概要
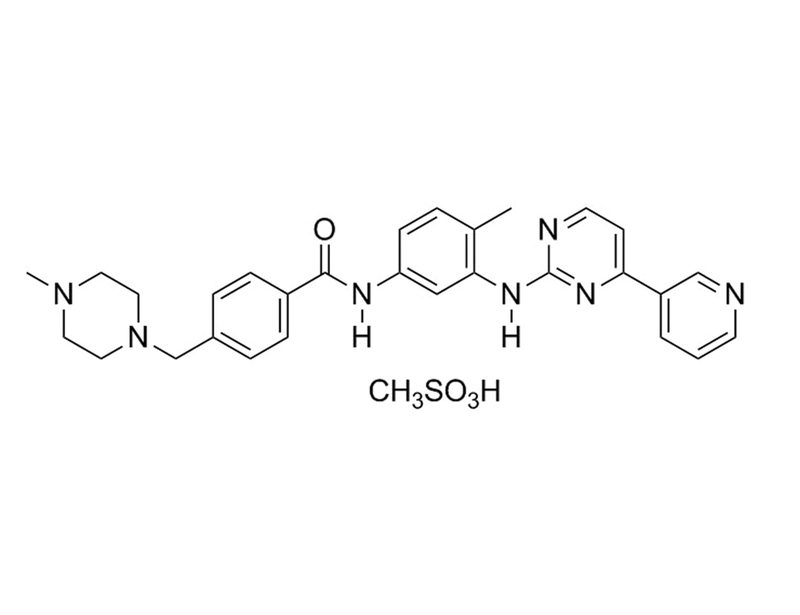
Imatinib mesylate is a first generation tyrosine kinase inhibitor that selectively targets certain tyrosine kinases, including ABL, platelet-derived growth factor receptor (PDGFR), and KIT (Druker, 2008; Müller).
DIFFERENTIATION
· Inhibits proliferation of primary cultured human mesenchymal stem cells, and promotes adipogenic over osteogenic differentiation (Fierro et al.).
· Induces osteoblast differentiation in cultured osteoblastic cells, and reduces osteoclastogenesis in mouse bone marrow cultures (O’Sullivan et al.).
CANCER RESEARCH
· In CML, Imatinib inhibits the oncoprotein BCR-ABL, the product of the Philadelphia chromosome gene fusion (Carroll et al.; Druker et al., 1996).
· Inhibits autonomous erythropoiesis in peripheral blood mononuclear cells isolated from patients with polycythemia vera (Oehler et al.).
DIFFERENTIATION
· Inhibits proliferation of primary cultured human mesenchymal stem cells, and promotes adipogenic over osteogenic differentiation (Fierro et al.).
· Induces osteoblast differentiation in cultured osteoblastic cells, and reduces osteoclastogenesis in mouse bone marrow cultures (O’Sullivan et al.).
CANCER RESEARCH
· In CML, Imatinib inhibits the oncoprotein BCR-ABL, the product of the Philadelphia chromosome gene fusion (Carroll et al.; Druker et al., 1996).
· Inhibits autonomous erythropoiesis in peripheral blood mononuclear cells isolated from patients with polycythemia vera (Oehler et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Imatinib (Mesylate) | 72532, 72534 | All | English |
| Safety Data Sheet | Imatinib (Mesylate) | 72532, 72534 | All | English |
数据及文献
Publications (7)
Current pharmaceutical design 2009 JAN
Imatinib and its successors--how modern chemistry has changed drug development.
Abstract
Abstract
Since protein kinases are frequently mutated or otherwise deregulated in human malignancies, they serve as a target for differentiating between tumor cells and normal tissues. Imatinib mesylat (IM), an inhibitor of the BCR-ABL tyrosine kinase was introduced in 2001 and has revolutionized the treatment of patients with chronic myeloid leukemia (CML). Since 2005 a second generation of tyrosine kinase inhibitors is to follow in Imatinib's footsteps: The development of these new small molecules was promoted by the identification of potential target kinases within the cellular signaling apparatus. Modern biochemical tools provide relevant amounts of these target kinases necessary for high throughput screening (HTS) campaigns and for elucidation of their 3-D structure by crystallography. Supported by computational chemistry the resulting data have enabled rational drug design. In this review low molecular weight inhibitors used for the CML treatment are summarized, pointing out their chemical similarities and differences.
Blood 2008 DEC
Translation of the Philadelphia chromosome into therapy for CML.
Abstract
Abstract
Throughout its history, chronic myeloid leukemia (CML) has set precedents for cancer research and therapy. These range from the identification of the first specific chromosomal abnormality associated with cancer to the development of imatinib as a specific, targeted therapy for the disease. The successful development of imatinib as a therapeutic agent for CML can be attributed directly to decades of scientific discoveries. These discoveries determined that the BCR-ABL tyrosine kinase is the critical pathogenetic event in CML and an ideal target for therapy. This was confirmed in clinical trials of imatinib, with imatinib significantly improving the long-term survival of patients with CML. Continuing in this tradition of scientific discoveries leading to improved therapies, the understanding of resistance to imatinib has rapidly led to strategies to circumvent resistance. Continued studies of hematologic malignancies will allow this paradigm of targeting molecular pathogenetic events to be applied to many additional hematologic cancers.
Journal of bone and mineral research 2007 NOV
Imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and inhibits osteoclastogenesis by both direct and stromal cell-dependent mechanisms.
Abstract
Abstract
UNLABELLED: Several lines of evidence suggest that imatinib may affect skeletal tissue. We show that inhibition by imatinib of PDGFR signaling in osteoblasts activates osteoblast differentiation and inhibits osteoblast proliferation and that imatinib inhibits osteoclastogenesis by both stromal cell-dependent and direct effects on osteoclast precursors. INTRODUCTION: Imatinib mesylate, an orally active inhibitor of the c-abl, c-kit, and platelet-derived growth factor receptor (PDGFR) tyrosine kinases, is in clinical use for the treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal cell tumors. Interruption of both c-kit and c-abl signaling in mice induces osteopenia, suggesting that imatinib might have adverse effects on the skeleton. However, biochemical markers of bone formation increase in patients with CML starting imatinib therapy, whereas bone resorption is unchanged, despite secondary hyperparathyroidism. We assessed the actions of imatinib on bone cells in vitro to study the cellular and molecular mechanism(s) underlying the skeletal effects we observed in imatinib-treated patients. MATERIALS AND METHODS: Osteoblast differentiation was assessed using a mineralization assay, proliferation by [(3)H]thymidine incorporation, and apoptosis by a TUNEL assay. Osteoclastogenesis was assessed using murine bone marrow cultures and RAW 264.7 cells. RT and multiplex PCR were performed on RNA prepared from human bone marrow samples, osteoblastic cells, and murine bone marrow cultures. Osteoprotegerin was measured by ELISA. RESULTS: The molecular targets of imatinib are expressed in bone cells. In vitro, imatinib increases osteoblast differentiation and prevents PDGF-induced inhibition of this process. Imatinib inhibits proliferation of osteoblast-like cells induced by serum and PDGF. In murine bone marrow cultures, imatinib inhibits osteoclastogenesis stimulated by 1,25-dihydroxyvitamin D(3) and partially inhibits osteoclastogenesis induced by RANKL and macrophage-colony stimulating factor. Imatinib partially inhibited osteoclastogenesis in RANKL-stimulated RAW-264.7 cells. Treatment with imatinib increases the expression of osteoprotegerin in bone marrow from patients with CML and osteoblastic cells. CONCLUSIONS: Taken together with recent in vivo data, these results suggest a role for the molecular targets of imatinib in bone cell function, that inhibition by imatinib of PDGFR signaling in osteoblasts activates bone formation, and that the antiresorptive actions of imatinib are mediated by both stromal cell-dependent and direct effects on osteoclast precursors.
Cell proliferation 2007 JUN
Inhibition of platelet-derived growth factor receptorbeta by imatinib mesylate suppresses proliferation and alters differentiation of human mesenchymal stem cells in vitro.
Abstract
Abstract
OBJECTIVES: Recent data show that Imatinib mesylate (IM) also affects haematopoietic stem cells (HSC), T lymphocytes and dendritic cells that do not harbour constitutively active tyrosine kinases. MATERIALS AND METHODS: We evaluated possible effects of IM on human bone marrow-derived mesenchymal stem cells (MSC) in vitro. RESULTS: Screening the activity of 42 receptor tyrosine kinases revealed an exclusive inhibition of platelet-derived growth factor receptorbeta (PDGFRbeta). Analysis of downstream targets of PDGFRbeta demonstrated IM-mediated reduction of Akt and Erk1/2 phosphorylation. Culture of MSC with IM led to the reversible development of perinuclear multi-vesicular bodies. The proliferation and clonogenicity of MSC were significantly reduced compared to control cultures. IM favoured adipogenic differentiation of MSC whereas osteogenesis was suppressed. The functional deficits described led to a 50% reduction in the support of clonogenic haematopoietic stem cells, cultured for 1 month on a monolayer of MSC with IM. CONCLUSION: In summary, inhibition of PDGFRbeta and downstream Akt and Erk signalling by IM has a significant impact on proliferation and differentiation of human MSC in vitro.
Blood 2003 SEP
Imatinib mesylate inhibits autonomous erythropoiesis in patients with polycythemia vera in vitro.
Abstract
Abstract
The overproduction of red blood cells in patients with polycythemia vera (PV) is reflected in vitro by the formation of erythroid burst-forming units (BFU-Es) in the absence of exogenous erythropoietin. In contrast to other myeloproliferative disorders, the molecular mechanism of PV is unknown and no specific chromosomal abnormality has been described. We speculated that imatinib mesylate may reverse the pathological overproduction of red cells by inhibition of autonomous erythropoiesis. In the present study, imatinib mesylate was found to either block or strongly inhibit autonomous BFU-E formation in vitro in all patients tested. Moreover, autonomous BFU-E growth was also markedly reduced by exposure of PV cells to imatinib mesylate prior to cultivation in semisolid medium. The profound effect of imatinib mesylate on autonomous erythropoiesis suggests the involvement of an as yet unidentified kinase in the pathogenesis of PV and should provide the rationale for a forthcoming clinical trial.
Blood 1997 DEC
CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins.
Abstract
Abstract
CGP 57148 is a compound of the 2-phenylaminopyrimidine class that selectively inhibits the tyrosine kinase activity of the ABL and the platelet-derived growth factor receptor (PDGFR) protein tyrosine kinases. We previously showed that CGP 57148 selectively kills p210BCR-ABL-expressing cells. To extend these observations, we evaluated the ability of CGP 57148 to inhibit other activated ABL tyrosine kinases, including p185BCR-ABL and TEL-ABL. In cell-based assays of ABL tyrosine phosphorylation, inhibition of ABL kinase activity was observed at concentrations similar to that reported for p210BCR-ABL. Consistent with the in vitro profile of this compound, the growth of cells expressing activated ABL protein tyrosine kinases was inhibited in the absence of exogenous growth factor. Growth inhibition was also observed with a p185BCR-ABL-positive acute lymphocytic leukemia (ALL) cell line generated from a Philadelphia chromosome-positive ALL patient. As CGP 57148 inhibits the PDGFR kinase, we also showed that cells expressing an activated PDGFR tyrosine kinase, TEL-PDGFR, are sensitive to this compound. Thus, this compound may be useful for the treatment of a variety of BCR-ABL-positive leukemias and for treatment of the subset of chronic myelomonocytic leukemia patients with a TEL-PDGFR fusion protein.

 网站首页
网站首页