概要
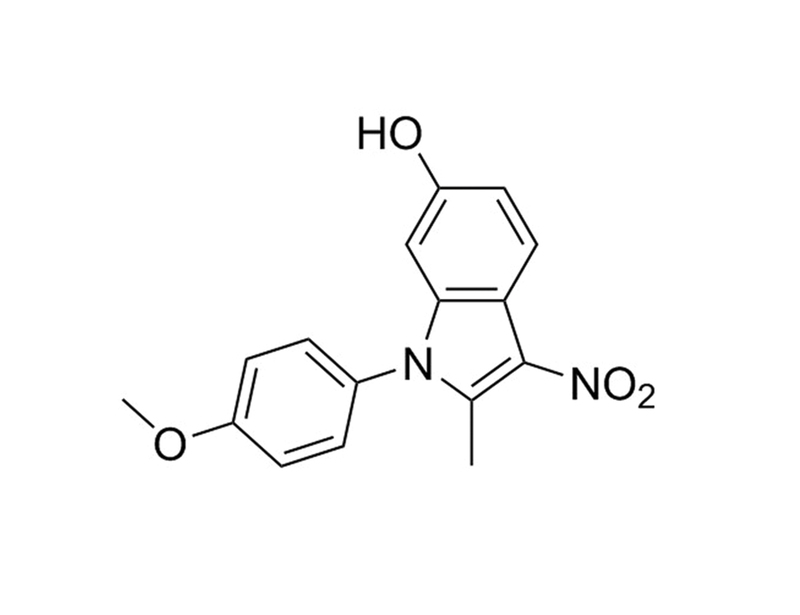
ID-8 is an indole derivative (Miyabayashi et al.) that inhibits members of the dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) family (Hasegawa et al.).
MAINTENANCE AND SELF-RENEWAL
· Enables maintenance of mouse embryonic stem (ES) cells in the absence of mouse embryonic fibroblast (MEF) feeder cells, serum, or LIF (Miyabayashi et al.).
· In combination with Wnt, supports human ES cell proliferation and survival, without FGF or TGFβ (Hasegawa et al.).
MAINTENANCE AND SELF-RENEWAL
· Enables maintenance of mouse embryonic stem (ES) cells in the absence of mouse embryonic fibroblast (MEF) feeder cells, serum, or LIF (Miyabayashi et al.).
· In combination with Wnt, supports human ES cell proliferation and survival, without FGF or TGFβ (Hasegawa et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | ID-8 | 72502 | All | English |
| Safety Data Sheet | ID-8 | 72502 | All | English |
数据及文献
Publications (2)
Stem Cells Translational Medicine 2011 DEC
Wnt Signaling Orchestration with a Small Molecule DYRK Inhibitor Provides Long-Term Xeno-Free Human Pluripotent Cell Expansion
Abstract
Abstract
An optimal culture system for human pluripotent stem cells should be fully defined and free of animal components. To date, most xeno-free culture systems require human feeder cells and/or highly complicated culture media that contain activators of the fibroblast growth factor (FGF) and transforming growth factor-β (TGFβ) signaling pathways, and none provide for replacement of FGF/TGFβ ligands with chemical compounds. The Wnt/β-catenin signaling pathway plays an important role in mouse embryonic stem cells in leukemia inhibitory factor-independent culture; however, the role of Wnt/β-catenin signaling in human pluripotent stem cell is still poorly understood and controversial because of the dual role of Wnts in proliferation and differentiation. Building on our previous investigations of small molecules modulating Wnt/β-catenin signaling in mouse embryonic stem cells, we identified a compound, ID-8, that could support Wnt-induced human embryonic stem cell proliferation and survival without differentiation. Dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) is the target of the small molecule ID-8. Its role in human pluripotent cell renewal was confirmed by DYRK knockdown in human embryonic stem cells. Using Wnt and the DYRK inhibitor ID-8, we have developed a novel and simple chemically defined xeno-free culture system that allows for long-term expansion of human pluripotent stem cells without FGF or TGFβ activation. These culture conditions do not include xenobiotic supplements, serum, serum replacement, or albumin. Using this culture system, we have shown that several human pluripotent cell lines maintained pluripotency (textgreater20 passages) and a normal karyotype and still retained the ability to differentiate into derivatives of all three germ layers. This Wnt-dependent culture system should provide a platform for complete replacement of growth factors with chemical compounds.
Bioscience, biotechnology, and biochemistry 2008 MAY
Indole derivatives sustain embryonic stem cell self-renewal in long-term culture.
Abstract
Abstract
Embryonic stem cells (ESCs), which have characteristics such as self-renewal, indefinite proliferation, and pluripotency, are thought to hold great promise for regenerative medicine. ESCs are generally cultured on mouse embryonic fibroblast (MEF) or MEF conditioned medium (MEF-CM). However, for therapeutic applications, it is preferable for ESCs to be cultured under chemically defined conditions. Here, we report synthetic compounds that allow expansion of undifferentiated mouse ESCs in the absence of MEF, Leukemia Inhibitory Factor (LIF), and Fetal Bovine Serum (FBS). ESCs cultured for more than 30 d in a serum-free medium supplemented with indole derivertives retained their characteristic morphology and expressed markers such as SSEA-1, OCT3/4, Rex-1, Sox2, and Nanog. They consistently differentiated into many types of cells, including neurons, muscle cells, and hepatocytes. These results indicate that our compounds provide a more efficient and safer large-scale culture system for pluripotent ESCs, and hence might contribute to the use of ESCs in therapeutic applications.

 网站首页
网站首页