概要
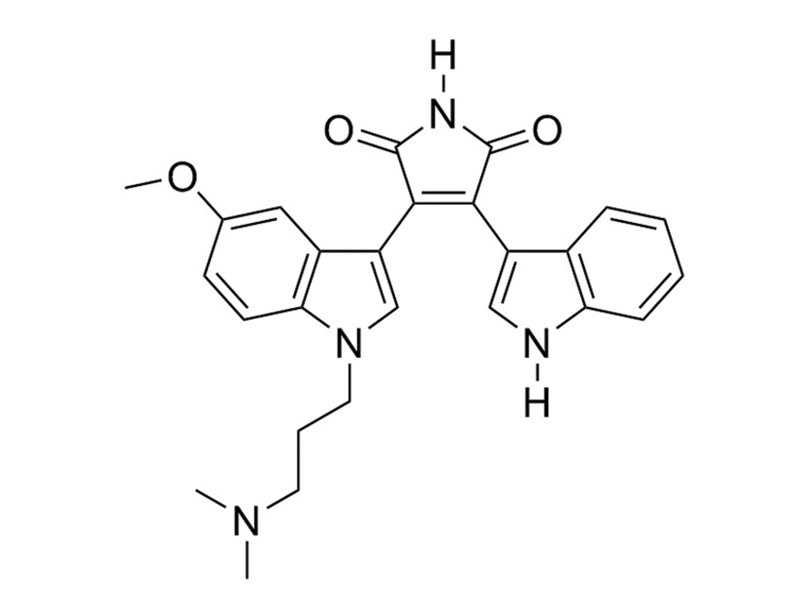
Gö6983 inhibits several isoforms of protein kinase C (PKC; IC₅₀ = 7, 7, 6, 10, 60, and 20,000 nM for PKCα, PKCβ, PKCγ, PKCδ, PKCζ, and PKCμ, respectively) (Gschwendt et al.).
REPROGRAMMING
· Enhances reprogramming of mouse embryonic fibroblasts to induced pluripotent stem cells (Dutta et al.).
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, RepSox, Forskolin, SP600125, Valproic Acid, and Y-27632 (Hu et al.).
MAINTENANCE AND SELF-RENEWAL
· Inhibits differentiation and maintains pluripotency in mouse embryonic stem cells (Dutta et al.).
· Enhances human ground state pluripotent stem cell viability and growth (Gafni et al.).
· Inhibits proliferation of human primary fetal bone cells (Krattinger et al.).
· Inhibits the formation of protoplatelets from megakaryocytes derived from adult mouse bone marrow (Williams et al.).
REPROGRAMMING
· Enhances reprogramming of mouse embryonic fibroblasts to induced pluripotent stem cells (Dutta et al.).
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, RepSox, Forskolin, SP600125, Valproic Acid, and Y-27632 (Hu et al.).
MAINTENANCE AND SELF-RENEWAL
· Inhibits differentiation and maintains pluripotency in mouse embryonic stem cells (Dutta et al.).
· Enhances human ground state pluripotent stem cell viability and growth (Gafni et al.).
· Inhibits proliferation of human primary fetal bone cells (Krattinger et al.).
· Inhibits the formation of protoplatelets from megakaryocytes derived from adult mouse bone marrow (Williams et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Gö6983 | 72462 | All | English |
| Safety Data Sheet | Gö6983 | 72462 | All | English |
数据及文献
Publications (4)
Cell stem cell 2015 AUG
Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules.
Abstract
Abstract
Neuronal conversion from human fibroblasts can be induced by lineage-specific transcription factors; however, the introduction of ectopic genes limits the therapeutic applications of such induced neurons (iNs). Here, we report that human fibroblasts can be directly converted into neuronal cells by a chemical cocktail of seven small molecules, bypassing a neural progenitor stage. These human chemical-induced neuronal cells (hciNs) resembled hiPSC-derived neurons and human iNs (hiNs) with respect to morphology, gene expression profiles, and electrophysiological properties. This approach was further applied to generate hciNs from familial Alzheimer's disease patients. Taken together, our transgene-free and chemical-only approach for direct reprogramming of human fibroblasts into neurons provides an alternative strategy for modeling neurological diseases and for regenerative medicine.
Platelets 2014 JAN
PKCα negatively regulates in vitro proplatelet formation and in vivo platelet production in mice.
Abstract
Abstract
Proplatelet formation is a part of the intricate process by which platelets are generated by their precursor cell, the megakaryocyte. The processes that drive megakaryocyte maturation and platelet production are however still not well understood. The protein kinase C (PKC) family of serine/threonine kinases has been demonstrated as an important regulator of megakaryocyte maturation and proplatelet formation, but little investigation has been made on the individual isoforms. We have previously shown, in mouse models, that PKCα plays a vital role in regulating platelet function, so in this study we aimed to investigate the role of PKCα in megakaryocyte function using the same Prkca(-)(/)(-) mice. We assessed the role of global PKC and specifically PKCα in proplatelet formation in vitro, analyzed polyploidy in Prkca(-)(/)(-)-derived megakaryocytes and followed platelet recovery in platelet-depleted Prkca(-)(/)(-) mice. We show reduced proplatelet formation in the presence of global PKC blockade. However, in the presence of a selective classical PKC isoform inhibitor, Go6976, proplatelet formation was conversely enhanced. PKCα null megakaryocytes also showed enhanced proplatelet formation, as well as a shift to greater polyploidy. In vivo, platelet production was enhanced in response to experimentally induced immune thrombocytopenia. In conclusion, our data indicate that classical PKC isoforms, and more specifically PKCα, are negative regulators of proplatelet formation. PKCα appears to negatively regulate endomitosis, with the enhanced polyploidy observed in Prkca(-)(/)(-)-derived megakaryocytes. In vivo, these observations may culminate in the observed ability of Prkca(-)(/)(-) mice to recover more rapidly from a thrombocytopenic insult.
Nature 2013 DEC
Derivation of novel human ground state naive pluripotent stem cells.
Abstract
Abstract
Mouse embryonic stem (ES) cells are isolated from the inner cell mass of blastocysts, and can be preserved in vitro in a naive inner-cell-mass-like configuration by providing exogenous stimulation with leukaemia inhibitory factor (LIF) and small molecule inhibition of ERK1/ERK2 and GSK3β signalling (termed 2i/LIF conditions). Hallmarks of naive pluripotency include driving Oct4 (also known as Pou5f1) transcription by its distal enhancer, retaining a pre-inactivation X chromosome state, and global reduction in DNA methylation and in H3K27me3 repressive chromatin mark deposition on developmental regulatory gene promoters. Upon withdrawal of 2i/LIF, naive mouse ES cells can drift towards a primed pluripotent state resembling that of the post-implantation epiblast. Although human ES cells share several molecular features with naive mouse ES cells, they also share a variety of epigenetic properties with primed murine epiblast stem cells (EpiSCs). These include predominant use of the proximal enhancer element to maintain OCT4 expression, pronounced tendency for X chromosome inactivation in most female human ES cells, increase in DNA methylation and prominent deposition of H3K27me3 and bivalent domain acquisition on lineage regulatory genes. The feasibility of establishing human ground state naive pluripotency in vitro with equivalent molecular and functional features to those characterized in mouse ES cells remains to be defined. Here we establish defined conditions that facilitate the derivation of genetically unmodified human naive pluripotent stem cells from already established primed human ES cells, from somatic cells through induced pluripotent stem (iPS) cell reprogramming or directly from blastocysts. The novel naive pluripotent cells validated herein retain molecular characteristics and functional properties that are highly similar to mouse naive ES cells, and distinct from conventional primed human pluripotent cells. This includes competence in the generation of cross-species chimaeric mouse embryos that underwent organogenesis following microinjection of human naive iPS cells into mouse morulas. Collectively, our findings establish new avenues for regenerative medicine, patient-specific iPS cell disease modelling and the study of early human development in vitro and in vivo.
Stem cells 2011 APR
Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance.
Abstract
Abstract
The intricate molecular mechanisms that regulate ESC pluripotency are incompletely understood. Prior research indicated that activation of the Janus kinase-signal transducer and activator of transcription (STAT3) pathway or inhibition of extracellular signal-regulated kinase/glycogen synthase kinase 3 (ERK/GSK3) signaling maintains mouse ESC (mESC) pluripotency. Here, we demonstrate that inhibition of protein kinase C (PKC) isoforms maintains mESC pluripotency without the activation of STAT3 or inhibition of ERK/GSK3 signaling pathways. Our analyses revealed that the atypical PKC isoform, PKCζ plays an important role in inducing lineage commitment in mESCs through a PKCζ-nuclear factor kappa-light-chain-enhancer of activated B cells signaling axis. Furthermore, inhibition of PKC isoforms permits derivation of germline-competent ESCs from mouse blastocysts and also facilitates reprogramming of mouse embryonic fibroblasts toward induced pluripotent stem cells. Our results indicate that PKC signaling is critical to balancing ESC self-renewal and lineage commitment.

 网站首页
网站首页