概要
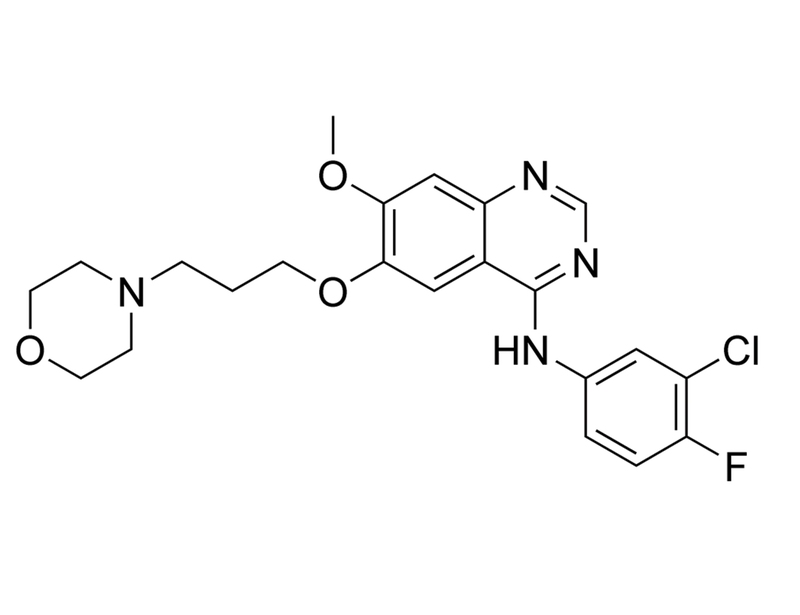
Gefitinib is a selective inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase that binds competitively in the ATP binding pocket with IC₅₀ values of 23 and 80 nM for A431 vulval squamous carcinoma cells and KB cells, respectively (Barker et al.).
CANCER RESEARCH
· Blocks proliferation in vitro and in mouse xenograft models, of multiple cancer cell types including colon, ovarian, and breast cancer cell lines (Ciardiello et al.).
· Induces apoptosis in the HaCaT human keratinocyte cell line via a c-Jun N-terminal kinase (JNK) activation, an EGFR-independent mechanism (Lu et al.).
CANCER RESEARCH
· Blocks proliferation in vitro and in mouse xenograft models, of multiple cancer cell types including colon, ovarian, and breast cancer cell lines (Ciardiello et al.).
· Induces apoptosis in the HaCaT human keratinocyte cell line via a c-Jun N-terminal kinase (JNK) activation, an EGFR-independent mechanism (Lu et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Gefitinib | 73162 | All | English |
| Safety Data Sheet | Gefitinib | 73162 | All | English |
数据及文献
Publications (3)
The British journal of dermatology 2011
Gefitinib-induced epidermal growth factor receptor-independent keratinocyte apoptosis is mediated by the JNK activation pathway.
Abstract
Abstract
BACKGROUND: Gefitinib (ZD1839) is a selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor with a significant antitumour effect on various cancers. Skin toxicity induced by gefitinib is common, and has been shown to be related to the inhibition of EGFR signalling pathways. However, other mechanisms may be involved in gefitinib-induced skin toxicity. OBJECTIVES: To study the possible EGFR-independent mechanisms of gefitinib-induced skin toxicity. METHODS: The human immortalized keratinocyte cell line HaCaT and human lung adenocarcinoma cell lines (A549 and PC9) were treated with different concentrations of gefitinib for 24, 48 and 72 h. Cell viability was measured by MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] after EGFR gene silencing. The signalling pathways were investigated by immunoblot analysis. Keratinocyte apoptosis was evaluated by nuclear condensation and flow cytometric analysis. RESULTS: Gefitinib maintained its cytotoxicity to HaCaT cells after EGFR gene silencing, indicating that an EGFR-independent mechanism exists. Increased phosphorylation of p38 mitogen-activated protein kinase and JNK by gefitinib was observed in a dose-dependent manner in HaCaT cells. The JNK inhibitor, SP600125, attenuated the gefitinib-induced cytotoxicity and apoptosis of HaCaT cells. Immunohistochemical examination of patient specimens showed an increased expression of phosphorylated JNK in lesional epidermis compared with nonlesional epidermis. CONCLUSIONS: Gefitinib can induce keratinocyte apoptosis through an EGFR-independent JNK activation pathway.
Journal of the National Cancer Institute 2007 APR
Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways.
Abstract
Abstract
BACKGROUND The epidermal growth factor receptor (EGFR) and Notch signaling pathways have been implicated in self-renewal of normal breast stem cells. We investigated the involvement of these signaling pathways in ductal carcinoma in situ (DCIS) of the breast. METHODS Samples of normal breast tissue (n = 15), pure DCIS tissue of varying grades (n = 35), and DCIS tissue surrounding an invasive cancer (n = 7) were used for nonadherent (i.e., mammosphere) culture. Mammosphere cultures were treated at day 0 with gefitinib (an EGFR inhibitor), DAPT (N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester) (a gamma-secretase inhibitor), or Notch 4-neutralizing antibody. Mammosphere-forming efficiency (MFE) was calculated by dividing the number of mammospheres of 60 microm or more formed by the number of single cells seeded and is expressed as a percentage. The Notch 1 intracellular domain (NICD) was detected immunohistochemically in paraffin-embedded DCIS tissue from 50 patients with at least 60 months of follow-up. All statistical tests were two-sided. RESULTS DCIS had a greater MFE than normal breast tissue (1.5% versus 0.5%, difference = 1%, 95% confidence interval [CI] = 0.62% to 1.25%, Ptextless.001). High-grade DCIS had a greater MFE than low-grade DCIS (1.6% versus 1.09%, difference = 0.51%, 95% CI = 0.07% to 0.94%, P = .01). The MFE of high-grade DCIS treated with gefitinib in the absence of exogenous EGF was lower than that of high-grade DCIS treated with mammosphere medium lacking gefitinib and exogenous EGF (0.56% versus 1.36%, difference 0.8%, 95% CI = 0.33% to 1.4%, P = .004). Increased Notch signaling as detected by NICD staining was associated with recurrence at 5 years (P = .012). DCIS MFE was reduced when Notch signaling was inhibited using either DAPT (0.89% versus 0.51%, difference = 0.38%, 95% CI = 0.2% to 0.6%, Ptextless.001) or a Notch 4-neutralizing antibody (0.97% versus 0.2%, difference = 0.77%, 95% CI = 0.52% to 1.0%, Ptextless.001). CONCLUSION We describe a novel primary culture technique for DCIS. Inhibition of the EGFR or Notch signaling pathways reduced DCIS MFE.
Bioorganic & medicinal chemistry letters 2001
Studies leading to the identification of ZD1839 (IRESSA): an orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer.
Abstract
Abstract
This paper describes the development of the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 from a lead series of 4-anilinoquinazoline compounds. ZD1839 has suitable properties for use as a clinically effective drug and shows activity against human tumours. In particular, the use of pharmacokinetic data in the development of ZD1839 is discussed.

 网站首页
网站首页