概要
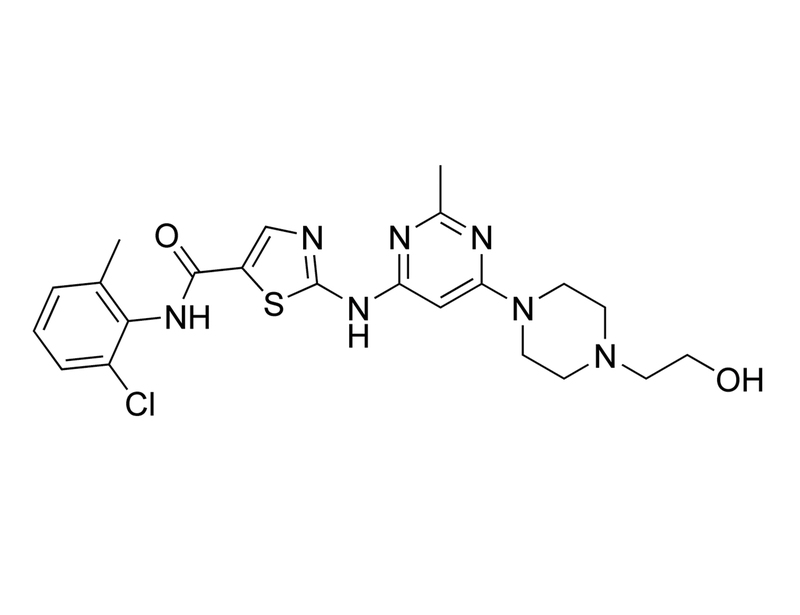
Dasatinib is a potent, ATP-competitive tyrosine kinase inhibitor. It is specific for SRC/ABL kinases, for example, ABL, SRC, LCK, and YES with IC₅₀ values of < 1.0, 0.5, 0.4 and 0.5 nM, respectively, and also demonstrates activity against KIT with an IC₅₀ = 5.0 nM (Lombardo et al.; Davis et al.) Dasatinib is a second-generation inhibitor of the oncogenic tyrosine kinase BCR-ABL with 325-fold more potency than imatinib, and is also able to inhibit imatinib-resistant BCR-ABL mutants (Tokarski et al.). It also inhibits a large number of other kinases (76 of 148 kinases tested) when screened at 10 μM (Carter et al.).
CANCER RESEARCH
· Inhibits proliferation in cell lines derived from chronic myeloid leukemia (CML), prostate, breast, and colon tumors (Lombardo et al.).
· Inhibits proliferation of cells with imatinib-resistant BCR-ABL mutations (Shah et al.).
· Inhibits tumor growth and development of lymph node metastases in orthotopic nude mouse models of prostate cancer (Park et al.).
· Induces cell-cycle arrest and apoptosis and decreases growth in thyroid cancer cells (Chan et al.).
· Inhibits production of extracellular matrix proteins in dermal fibroblasts and prevents development of bleomycin-challenge-induced fibrosis in mice (Distler & Distler; Akhmetshina et al.).
CANCER RESEARCH
· Inhibits proliferation in cell lines derived from chronic myeloid leukemia (CML), prostate, breast, and colon tumors (Lombardo et al.).
· Inhibits proliferation of cells with imatinib-resistant BCR-ABL mutations (Shah et al.).
· Inhibits tumor growth and development of lymph node metastases in orthotopic nude mouse models of prostate cancer (Park et al.).
· Induces cell-cycle arrest and apoptosis and decreases growth in thyroid cancer cells (Chan et al.).
· Inhibits production of extracellular matrix proteins in dermal fibroblasts and prevents development of bleomycin-challenge-induced fibrosis in mice (Distler & Distler; Akhmetshina et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Dasatinib | 73082, 73084 | All | English |
| Safety Data Sheet | Dasatinib | 73082, 73084 | All | English |
数据及文献
Publications (9)
Clinical cancer research : an official journal of the American Association for Cancer Research 2012
Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis.
Abstract
Abstract
PURPOSE: There are no effective therapies for patients with poorly differentiated papillary thyroid cancer (PTC) or anaplastic thyroid cancer (ATC), and metastasis to the bone represents a significantly worse prognosis. Src family kinases (SFKs) are overexpressed and activated in numerous tumor types and have emerged as a promising therapeutic target, especially in relation to metastasis. We recently showed that Src is overexpressed and activated in thyroid cancer. We therefore tested whether inhibition of Src with dasatinib (BMS-354825) blocks thyroid cancer growth and metastasis. EXPERIMENTAL DESIGN: The effects of dasatinib on thyroid cancer growth, signaling, cell cycle, and apoptosis were evaluated in vitro. The therapeutic efficacy of dasatinib was further tested in vivo using an orthotopic and a novel experimental metastasis model. Expression and activation of SFKs in thyroid cancer cells was characterized, and selectivity of dasatinib was determined using an Src gatekeeper mutant. RESULTS: Dasatinib treatment inhibited Src signaling, decreased growth, and induced cell-cycle arrest and apoptosis in a subset of thyroid cancer cells. Immunoblotting showed that c-Src and Lyn are expressed in thyroid cancer cells and that c-Src is the predominant SFK activated. Treatment with dasatinib blocked PTC tumor growth in an orthotopic model by more than 90% (P = 0.0014). Adjuvant and posttreatment approaches with dasatinib significantly inhibited metastasis (P = 0.016 and P = 0.004, respectively). CONCLUSION: These data provide the first evidence that Src is a central mediator of thyroid cancer growth and metastasis, indicating that Src inhibitors may have a higher therapeutic efficacy in thyroid cancer, as both antitumor and antimetastatic agents.
Nature biotechnology 2011
Comprehensive analysis of kinase inhibitor selectivity.
Abstract
Abstract
We tested the interaction of 72 kinase inhibitors with 442 kinases covering textgreater80% of the human catalytic protein kinome. Our data show that, as a class, type II inhibitors are more selective than type I inhibitors, but that there are important exceptions to this trend. The data further illustrate that selective inhibitors have been developed against the majority of kinases targeted by the compounds tested. Analysis of the interaction patterns reveals a class of 'group-selective' inhibitors broadly active against a single subfamily of kinases, but selective outside that subfamily. The data set suggests compounds to use as tools to study kinases for which no dedicated inhibitors exist. It also provides a foundation for further exploring kinase inhibitor biology and toxicity, as well as for studying the structural basis of the observed interaction patterns. Our findings will help to realize the direct enabling potential of genomics for drug development and basic research about cellular signaling.
Cancer research 2008
Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model.
Abstract
Abstract
Aberrant expression and/or activity of members of the Src family of nonreceptor protein tyrosine kinases (SFK) are commonly observed in progressive stages of human tumors. In prostate cancer, two SFKs (Src and Lyn) have been specifically implicated in tumor growth and progression. However, there are no data in preclinical models demonstrating potential efficacy of Src inhibitors against prostate cancer growth and/or metastasis. In this study, we used the small molecule SFK/Abl kinase inhibitor dasatinib, currently in clinical trials for solid tumors, to examine in vitro and in vivo effects of inhibiting SFKs in prostate tumor cells. In vitro, dasatinib inhibits both Src and Lyn activity, resulting in decreased cellular proliferation, migration, and invasion. In orthotopic nude mouse models, dasatinib treatment effectively inhibits expression of activated SFKs, resulting in inhibition of both tumor growth and development of lymph node metastases in both androgen-sensitive and androgen-resistant tumors. In primary tumors, SFK inhibition leads to decreased cellular proliferation (determined by immunohistochemistry for proliferating cell nuclear antigen). In vitro, small interfering RNA (siRNA)-mediated inhibition of Lyn affects cellular proliferation; siRNA inhibition of Src affects primarily cellular migration. Therefore, we conclude that SFKs are promising therapeutic targets for treatment of human prostate cancer and that Src and Lyn activities affect different cellular functions required for prostate tumor growth and progression.
Rheumatology (Oxford, England) 2008
Intracellular tyrosine kinases as novel targets for anti-fibrotic therapy in systemic sclerosis.
Abstract
Abstract
Tissue fibrosis is a major cause of death in SSc, but therapies that target selectively fibrosis are not yet available for routine clinical use. Recent pre-clinical studies suggest that selective tyrosine kinase inhibitors that target c-Abl, PDGF receptor or Src kinases might be promising targets for anti-fibrotic approaches. Dual inhibition of c-Abl and PDGF receptor by imatinib and nilotinib, and inhibition of Src kinases either selectively by SU6656 or in combination with c-Abl and PDGF by dasatinib exerted potent anti-fibrotic effects. Imatinib, nilotinib, dasatinib and SU6656 reduced dose-dependently the synthesis of extracellular matrix protein in human dermal fibroblasts in vitro and prevented fibrosis in the mouse model of bleomycin-induced skin fibrosis. Clinical data from patients with chronic myelogenous leukaemia suggest that imatinib, nilotinib and dasatinib are well tolerated. Based on the promising pre-clinical data, imatinib is currently evaluated in clinical trials for the treatment of fibrosis in SSc and trials with other tyrosine kinase inhibitors are in preparation.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2008
Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis.
Abstract
Abstract
Abelson kinase (c-abl) and platelet-derived growth factor (PDGF) are key players in the pathogenesis of systemic sclerosis (SSc). The aim of the present study was to evaluate the antifibrotic potential of dasatinib and nilotinib, 2 novel inhibitors of c-abl and PDGF, which are well tolerated and have recently been approved. Dasatinib and nilotinib dose-dependently reduced the mRNA and protein levels of extracellular matrix proteins in human stimulated dermal fibroblasts from SSc patients (IC(50) of 0.5-2.0 nM for dasatinib and 0.8-2.5 nM for nilotinib). In a mouse model of bleomycin-induced dermal fibrosis, dasatinib and nilotinib potently reduced the dermal thickness, the number of myofibroblasts, and the collagen content of the skin in a dose-dependent manner at well-tolerated doses. These data indicate that dasatinib and nilotinib potently inhibit the synthesis of extracellular matrix in vitro and in vivo at biologically relevant concentrations. Thus, we provide the first evidence that dasatinib and nilotinib might be promising drugs for the treatment of patients with SSc.
Cancer research 2006 JUN
The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants.
Abstract
Abstract
Chronic myeloid leukemia (CML) is caused by the constitutively activated tyrosine kinase breakpoint cluster (BCR)-ABL. Current frontline therapy for CML is imatinib, an inhibitor of BCR-ABL. Although imatinib has a high rate of clinical success in early phase CML, treatment resistance is problematic, particularly in later stages of the disease, and is frequently mediated by mutations in BCR-ABL. Dasatinib (BMS-354825) is a multitargeted tyrosine kinase inhibitor that targets oncogenic pathways and is a more potent inhibitor than imatinib against wild-type BCR-ABL. It has also shown preclinical activity against all but one of the imatinib-resistant BCR-ABL mutants tested to date. Analysis of the crystal structure of dasatinib-bound ABL kinase suggests that the increased binding affinity of dasatinib over imatinib is at least partially due to its ability to recognize multiple states of BCR-ABL. The structure also provides an explanation for the activity of dasatinib against imatinib-resistant BCR-ABL mutants.

 网站首页
网站首页