概要
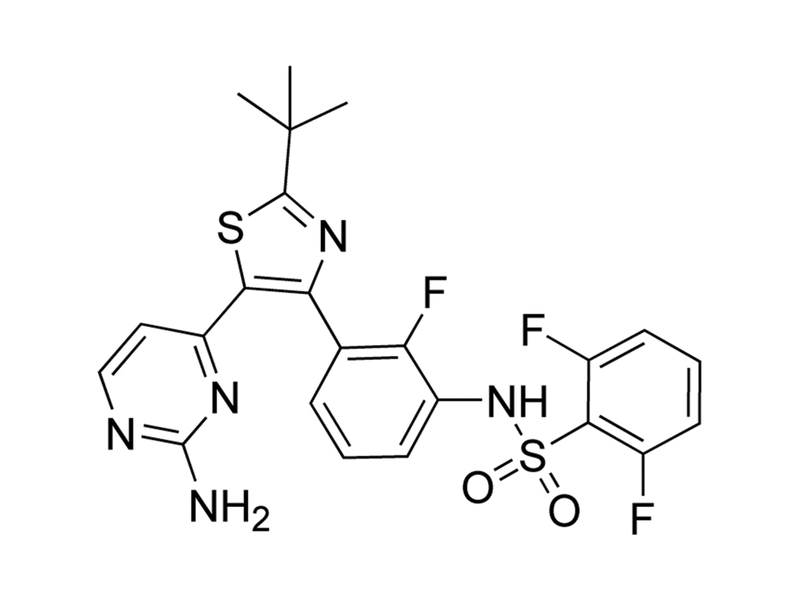
Dabrafenib is a reversible, selective, and ATP-competitive inhibitor of B-RAF with higher potency towards V600E mutant B-RAF over wild-type B-RAF or C-RAF, with IC₅₀ values of 0.8, 3.2 and 5 nM, respectively (Laquerre et al.; Menzies et al.).
CANCER RESEARCH
· Decreases ERK phosphorylation and inhibits cell proliferation in cells expressing the B-RAF mutation V600E. Prevents tumor growth in mouse xenograft models of B-RAF(V600E) human melanoma and colon cancer (Rheault et al.).
DISEASE MODELING
· Alleviates acetaminophen-induced liver injury through distinct nanomolar inhibition of receptor-interacting protein (RIP3) in mice (Li et al.).
CANCER RESEARCH
· Decreases ERK phosphorylation and inhibits cell proliferation in cells expressing the B-RAF mutation V600E. Prevents tumor growth in mouse xenograft models of B-RAF(V600E) human melanoma and colon cancer (Rheault et al.).
DISEASE MODELING
· Alleviates acetaminophen-induced liver injury through distinct nanomolar inhibition of receptor-interacting protein (RIP3) in mice (Li et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Dabrafenib | 73072, 73074 | All | English |
| Safety Data Sheet | Dabrafenib | 73072, 73074 | All | English |
数据及文献
Publications (3)
Cell death & disease 2014
The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury.
Abstract
Abstract
Receptor-interacting protein (RIP)3 is a critical regulator of necroptosis and has been demonstrated to be associated with various diseases, suggesting that its inhibitors are promising in the clinic. However, there have been few RIP3 inhibitors reported as yet. B-Raf(V600E) inhibitors are an important anticancer drug class for metastatic melanoma therapy. In this study, we found that 6 B-Raf inhibitors could inhibit RIP3 enzymatic activity in vitro. Among them, dabrafenib showed the most potent inhibition on RIP3, which was achieved by its ATP-competitive binding to the enzyme. Dabrafenib displayed highly selective inhibition on RIP3 over RIP1, RIP2 and RIP5. Moreover, only dabrafenib rescued cells from RIP3-mediated necroptosis induced by the necroptosis-induced combinations, that is, tumor necrosis factor (TNF)α, TNF-related apoptosis-inducing ligand or Fas ligand plus Smac mimetic and the caspase inhibitor z-VAD. Dabrafenib decreased the RIP3-mediated Ser358 phosphorylation of mixed lineage kinase domain-like protein (MLKL) and disrupted the interaction between RIP3 and MLKL. Notably, RIP3 inhibition of dabrafenib appeared to be independent of its B-Raf inhibition. Dabrafenib was further revealed to prevent acetaminophen-induced necrosis in normal human hepatocytes, which is considered to be mediated by RIP3. In acetaminophen-overdosed mouse models, dabrafenib was found to apparently ease the acetaminophen-caused liver damage. The results indicate that the anticancer B-Raf(V600E) inhibitor dabrafenib is a RIP3 inhibitor, which could serve as a sharp tool for probing the RIP3 biology and as a potential preventive or therapeutic agent for RIP3-involved necroptosis-related diseases such as acetaminophen-induced liver damage.
ACS medicinal chemistry letters 2013 MAR
Discovery of Dabrafenib: A Selective Inhibitor of Raf Kinases with Antitumor Activity against B-Raf-Driven Tumors.
Abstract
Abstract
Hyperactive signaling of the MAP kinase pathway resulting from the constitutively active B-Raf(V600E) mutated enzyme has been observed in a number of human tumors, including melanomas. Herein we report the discovery and biological evaluation of GSK2118436, a selective inhibitor of Raf kinases with potent in vitro activity in oncogenic B-Raf-driven melanoma and colorectal carcinoma cells and robust in vivo antitumor and pharmacodynamic activity in mouse models of B-Raf(V600E) human melanoma. GSK2118436 was identified as a development candidate, and early clinical results have shown significant activity in patients with B-Raf mutant melanoma.
Drug design, development and therapy 2012
Dabrafenib and its potential for the treatment of metastatic melanoma.
Abstract
Abstract
The purpose of this study is to review the development of BRAF inhibitors, with emphasis on the trials conducted with dabrafenib (GSK2118436) and the evolving role of dabrafenib in treatment for melanoma patients. Fifty percent of cutaneous melanomas have mutations in BRAF, resulting in elevated activity of the mitogen-activated protein kinase signaling pathway. Dabrafenib inhibits the mutant BRAF (BRAF(mut)) protein in melanomas with BRAF(V600E) and BRAF(V600K) genotypes. BRAF(V600E) metastatic melanoma patients who receive dabrafenib treatment exhibit high clinical response rates and compared with dacarbazine chemotherapy, progression-free survival. Efficacy has also been demonstrated in BRAF(V600K) patients and in those with brain metastases. Dabrafenib has a generally mild and manageable toxicity profile. Cutaneous squamous cell carcinomas and pyrexia are the most significant adverse effects. Dabrafenib appears similar to vemurafenib with regard to efficacy but it is associated with less toxicity. It is expected that new combinations of targeted drugs, such as the combination of dabrafenib and trametinib (GSK1120212, a MEK inhibitor), will provide higher response rates and more durable clinical benefit than dabrafenib monotherapy.

 网站首页
网站首页