概要
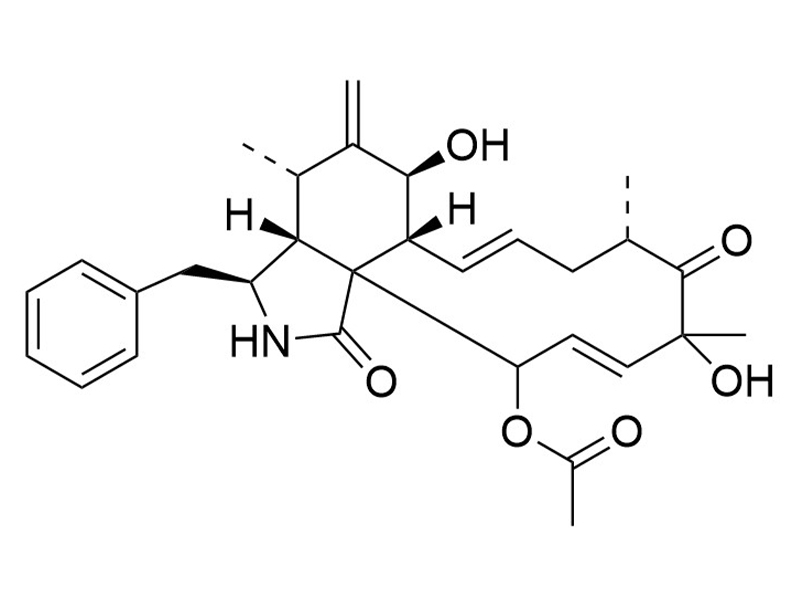
Cytochalasin D is a fungal toxin and a cell-permeable inhibitor of actin polymerization (Brenner & Korn). It also partially blocks HIV-1 release from infected human embryonic stem cells and lymphoma cells (Sasaki et al.).
DIFFERENTIATION
· Reduces cell viability and inhibits osteoblastic cells differentiation in human stromal stem cells (Chen et al.).
CANCER RESEARCH
· Binds actin filament and prevents tumor migration in human breast and lung cancer cells (Hayot et al.).
DIFFERENTIATION
· Reduces cell viability and inhibits osteoblastic cells differentiation in human stromal stem cells (Chen et al.).
CANCER RESEARCH
· Binds actin filament and prevents tumor migration in human breast and lung cancer cells (Hayot et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Cytochalasin D | 100-0556, 100-0557 | All | English |
| Safety Data Sheet | Cytochalasin D | 100-0556, 100-0557 | All | English |
数据及文献
Publications (4)
Stem cell research 2015 sep
Inhibiting actin depolymerization enhances osteoblast differentiation and bone formation in human stromal stem cells.
Abstract
Abstract
Remodeling of the actin cytoskeleton through actin dynamics is involved in a number of biological processes, but its role in human stromal (skeletal) stem cells (hMSCs) differentiation is poorly understood. In the present study, we demonstrated that stabilizing actin filaments by inhibiting gene expression of the two main actin depolymerizing factors (ADFs): Cofilin 1 (CFL1) and Destrin (DSTN) in hMSCs, enhanced cell viability and differentiation into osteoblastic cells (OB) in vitro, as well as heterotopic bone formation in vivo. Similarly, treating hMSC with Phalloidin, which is known to stabilize polymerized actin filaments, increased hMSCs viability and OB differentiation. Conversely, Cytocholasin D, an inhibitor of actin polymerization, reduced cell viability and inhibited OB differentiation of hMSC. At a molecular level, preventing Cofilin phosphorylation through inhibition of LIM domain kinase 1 (LIMK1) decreased cell viability and impaired OB differentiation of hMSCs. Moreover, depolymerizing actin reduced FAK, p38 and JNK activation during OB differentiation of hMSCs, while polymerizing actin enhanced these signaling pathways. Our results demonstrate that the actin dynamic reassembly and Cofilin phosphorylation loop is involved in the control of hMSC proliferation and osteoblasts differentiation.
Toxicology and applied pharmacology 2006 feb
Characterization of the activities of actin-affecting drugs on tumor cell migration.
Abstract
Abstract
Metastases kill 90{\%} of cancer patients. It is thus a major challenge in cancer therapy to inhibit the spreading of tumor cells from primary tumor sites to those particular organs where metastases are likely to occur. Whereas the actin cytoskeleton is a key component involved in cell migration, agents targeting actin dynamics have been relatively poorly investigated. Consequently, valuable in vitro pharmacological tools are needed to selectively identify this type of agent. In response to the absence of any standardized process, the present work aims to develop a multi-assay strategy for screening actin-affecting drugs with anti-migratory potentials. To validate our approach, we used two cancer cell lines (MCF7 and A549) and three actin-affecting drugs (cytochalasin D, latrunculin A, and jasplakinolide). We quantified the effects of these drugs on the kinetics of actin polymerization in tubes (by means of spectrofluorimetry) and on the dynamics of actin cytoskeletons within whole cells (by means of fluorescence microscopy). Using quantitative videomicroscopy, we investigated the actual effects of the drugs on cell motility. Finally, the combined drug effects on cell motility and cell growth were evaluated by means of a scratch-wound assay. While our results showed concordant drug-induced effects on actin polymerization occurring in vitro in test tubes and within whole cells, the whole cell assay appeared more sensitive than the tube assay. The inhibition of actin polymerization induced by cytochalasin D was paralleled by a decrease in cell motility for both cell types. In the case of jasplakinolide, which induces actin polymerization, while it significantly enhanced the locomotion of the A549 cells, it significantly inhibited that of the MCF-7 ones. All these effects were confirmed by means of the scratch-wound assay except of the jasplakinolide-induced effects on MCF-7 cell motility. These later seemed compensated by an additional effect occurring during wound recolonization (possibly acting on the cell growth features). In conclusion, the use of multi-assays with different levels of sophistication and biological relevance is recommended in the screening of new actin-affecting drugs with potentially anti-migratory effects.
Proceedings of the National Academy of Sciences of the United States of America 1995 mar
Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells.
Abstract
Abstract
We examined the potential role of myosin and actin in the release of human immunodeficiency virus type 1 (HIV-1) from infected cells. Wortmannin (100 nM to 5 microM), an effective inhibitor of myosin light chain kinase, blocked the release of HIV-1 from infected T-lymphoblastoid and monocytoid cells in a concentration-dependent manner. Cytochalasin D, a reagent that disrupts the equilibrium between monomeric and polymeric actin, also partially inhibited the release of HIV-1 from the infected cells. At the budding stage, myosin and HIV-1 protein were detected in the same areas on the plasma membrane by using dual-label immunofluorescence microscopy and immunoelectron microscopy. In the presence of 5 microM wortmannin, viral components were observed on the plasma membrane by using immunofluorescence microscopy and electron microscopy, implying that wortmannin did not disturb the transport of viral proteins to the plasma membrane but rather inhibited budding.
The Journal of biological chemistry 1980 feb
The effects of cytochalasins on actin polymerization and actin ATPase provide insights into the mechanism of polymerization.
Abstract
Abstract
Substoichiometric concentrations of cytochalasin D inhibited the rate of polymerization of actin in 0.5 mM MgCl2, increased its critical concentration and lowered its steady state viscosity. Stoichiometric concentrations of cytochalasin D in 0.5 mM MgCl2 and even substoichiometric concentrations of cytochalasin D in 30 mM KCl, however, accelerated the rate of actin polymerization, although still lowering the final steady state viscosity. Cytochalasin B, at all concentrations in 0.5 mM MgCl2 or in 30 mM KCl, accelerated the rate of polymerization and lowered the final steady state viscosity. In 0.5 mM MgCl2, cytochalasin D uncoupled the actin ATPase activity from actin polymerization, increasing the ATPase rate by at least 20 times while inhibiting polymerization. Cytochalasin B had a very much lower stimulating effect. Neither cytochalasin D nor B affected the actin ATPase activity in 30 mM KCl. The properties of cytochalasin E were intermediate between those of cytochalasin D and B. Cytochalasin D also stimulated the ATPase activity of monomeric actin in the absence of MgCl2 and KCl and, to a much greater extent, stimulated the ATPase activity of monomeric actin below its critical concentration in 0.5 mM MgCl2. Both above and below its critical concentration and in the presence and absence of cytochalasin D, the initial rate of actin ATPase activity, when little or no polymerization had occurred, was directly proportional to the actin concentration and, therefore, apparently was independent of actin-actin interactions. To rationalize all these data, a working model has been proposed in which the first step of actin polymerization is the conversion of monomeric actin-bound ATP, A . ATP, to monomeric actin-bound ADP and Pi, A* . ADP . Pi, which, like the preferred growing end of an actin filament, can bind cytochalasins.

 网站首页
网站首页