概要
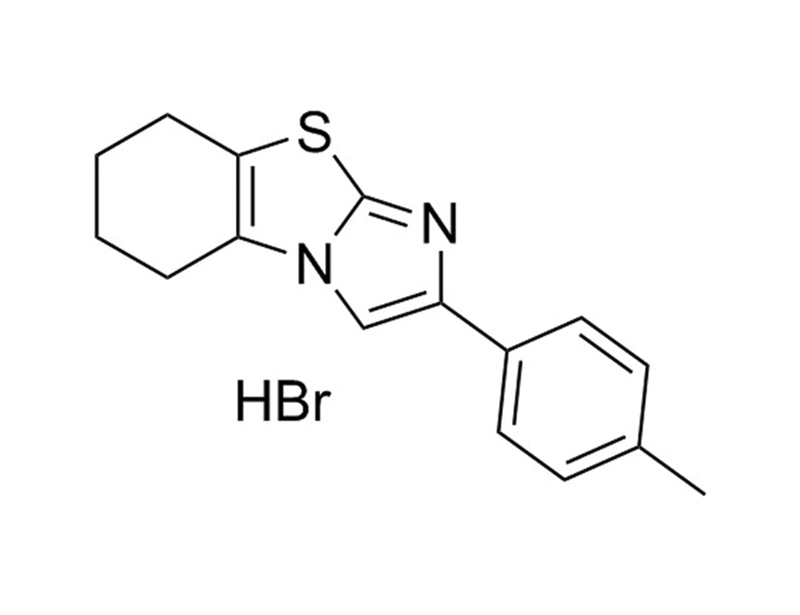
Cyclic Pifthrin-Alpha is a cell-permeable and reversible inhibitor of p53-mediated apoptosis and p53-dependent gene transcription. It is a more stable and less cytotoxic analog of the non-cyclic form of pifithrin-alpha. Cyclic Pifithrin-Alpha has also been reported to activate the aryl hydrocarbon receptor. (Fernandez-Cruz et al., Gary & Jensen, Komarov et al.)
REPROGRAMMING
· Increases efficiency of reprogramming mouse embryonic fibroblasts to induced pluripotent stem cells (Liao et al.).
MAINTENANCE
· Reduces UV-induced apoptosis of mouse embryonic stem cells (Qin et al.).
· Increases the numbers of mouse hematopoietic stem and progenitor cells in vivo and in vitro, also decreases the radiation-induced death of these cells (Leonova et al.).
REPROGRAMMING
· Increases efficiency of reprogramming mouse embryonic fibroblasts to induced pluripotent stem cells (Liao et al.).
MAINTENANCE
· Reduces UV-induced apoptosis of mouse embryonic stem cells (Qin et al.).
· Increases the numbers of mouse hematopoietic stem and progenitor cells in vivo and in vitro, also decreases the radiation-induced death of these cells (Leonova et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Cyclic Pifithrin-Alpha (Hydrobromide) | 72062, 72064 | All | English |
| Safety Data Sheet | Cyclic Pifithrin-Alpha (Hydrobromide) | 72062, 72064 | All | English |
数据及文献
Publications (6)
Molecular therapy : the journal of the American Society of Gene Therapy 2013 JUN
Inhibition of PTEN tumor suppressor promotes the generation of induced pluripotent stem cells.
Abstract
Abstract
Induced pluripotent stem cells (iPSCs) can be generated from patients with specific diseases by the transduction of reprogramming factors and can be useful as a cell source for cell transplantation therapy for various diseases with impaired organs. However, the low efficiency of iPSC derived from somatic cells (0.01-0.1%) is one of the major problems in the field. The phosphoinositide 3-kinase (PI3K) pathway is thought to be important for self-renewal, proliferation, and maintenance of embryonic stem cells (ESCs), but the contribution of this pathway or its well-known negative regulator, phosphatase, and tensin homolog deleted on chromosome ten (Pten), to somatic cell reprogramming remains largely unknown. Here, we show that activation of the PI3K pathway by the Pten inhibitor, dipotassium bisperoxo(5-hydroxypyridine-2-carboxyl)oxovanadate, improves the efficiency of germline-competent iPSC derivation from mouse somatic cells. This simple method provides a new approach for efficient generation of iPSCs.
Life sciences 2011 APR
Biological and chemical studies on aryl hydrocarbon receptor induction by the p53 inhibitor pifithrin-α and its condensation product pifithrin-β.
Abstract
Abstract
AIMS Pifithrin α (PFTα), an inhibitor of the p53 protein, is regarded as a lead compound for cancer and neurodegenerative disease therapy. There is some evidence that this compound activates the aryl hydrocarbon receptor (AhR) in a complete independent way of the p53 inhibition and that it is easily converted to its condensation product pifithrin β (PFTβ). The aim of this study was to explore the ability of PFTα and of PFTβ to induce a variety of AhR mediated processes. MAIN METHODS Computational analysis using quantum chemical calculations and chemical analysis have been used to study the conformation of the compounds as well as the cyclization reaction. The AhR mediated processes of these compounds have been studied in a rainbow trout cell line (RTG-2) and in a rat hepatoma cell line (H4IIE). KEY FINDINGS PFTα molecule could not take a planar conformation required for AhR activation whereas PFTβ showed a conformation similar to those of the prototypical AhR ligand β-naphthoflavone. In both cell lines, PFTα and PFTβ provoked different responses related with AhR activation. However, when cyclization of PFTα to PFTβ was hampered by acetylation of the exocyclic nitrogen, all these responses were not observed. These results lead to the conclusion that the activation of the AhR is probably caused by PFTβ instead of PFTα. SIGNIFICANCE Since PFTα is a promising compound for the development of new pharmaceuticals inhibiting p53, the chemical instability of this compound as well as the capacity of its transformation product should be taken into account.
Cell cycle (Georgetown, Tex.) 2010 APR
A small molecule inhibitor of p53 stimulates amplification of hematopoietic stem cells but does not promote tumor development in mice.
Abstract
Abstract
It has been shown that genetic inhibition of p53 leads to enhanced proliferation of hematopoietic stem cells (HSCs). This could, in theory, contribute to the increased frequency of tumor development observed in p53-deficient mice and humans. In our previous work, we identified chemical p53 inhibitors (PFTs) that suppress the transactivation function of p53 and protect cultured cells and mice from death induced by gamma irradiation (IR). Here we found that when applied to bone marrow cells in vitro or injected into mice, PFTb impeded IR-induced reduction of hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) population sizes. In addition, we showed that PFTb stimulated HSC and HPC proliferation in the absence of IR in vitro and in vivo and mobilized HSCs to the peripheral blood. Importantly, however, PFTb treatment did not affect the timing or frequency of tumor development in irradiated p53 heterozygous mice used as a model for determination of carcinogenicity. Thus, although PFTb administration led to increased numbers of HSCs and HPCs, it was not carcinogenic in mice. These findings suggest that chemical p53 inhibitors may be clinically useful as safe and effective stimulators of hematopoiesis.
The Journal of biological chemistry 2007 FEB
Regulation of apoptosis and differentiation by p53 in human embryonic stem cells.
Abstract
Abstract
The essentially infinite expansion potential and pluripotency of human embryonic stem cells (hESCs) makes them attractive for cell-based therapeutics. In contrast to mouse embryonic stem cells (mESCs), hESCs normally undergo high rates of spontaneous apoptosis and differentiation, making them difficult to maintain in culture. Here we demonstrate that p53 protein accumulates in apoptotic hESCs induced by agents that damage DNA. However, despite the accumulation of p53, it nevertheless fails to activate the transcription of its target genes. This inability of p53 to activate its target genes has not been observed in other cell types, including mESCs. We further demonstrate that p53 induces apoptosis of hESCs through a mitochondrial pathway. Reducing p53 expression in hESCs in turn reduces both DNA damage-induced apoptosis as well as spontaneous apoptosis. Reducing p53 expression also reduces spontaneous differentiation and slows the differentiation rate of hESCs. Our studies reveal the important roles of p53 as a critical mediator of human embryonic stem cells survival and differentiation.
Science (New York, N.Y.) 1999 SEP
A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy.
Abstract
Abstract
Chemotherapy and radiation therapy for cancer often have severe side effects that limit their efficacy. Because these effects are in part determined by p53-mediated apoptosis, temporary suppression of p53 has been suggested as a therapeutic strategy to prevent damage of normal tissues during treatment of p53-deficient tumors. To test this possibility, a small molecule was isolated for its ability to reversibly block p53-dependent transcriptional activation and apoptosis. This compound, pifithrin-alpha, protected mice from the lethal genotoxic stress associated with anticancer treatment without promoting the formation of tumors. Thus, inhibitors of p53 may be useful drugs for reducing the side effects of cancer therapy and other types of stress associated with p53 induction.
Molecular pharmaceutics JAN
The p53 inhibitor pifithrin-alpha forms a sparingly soluble derivative via intramolecular cyclization under physiological conditions.
Abstract
Abstract
The transcription factor p53 coordinates cell cycle arrest and apoptosis in response to DNA damage. Pifithrin-alpha (PFT-alpha) is a small molecule inhibitor of p53 activity that is frequently used in cell culture studies of p53 function. Here we report an investigation of the stability of this compound. PFT-alpha rapidly converts to a planar tricyclic derivative, with a half-life of 4.2 h under physiological conditions. This spontaneous conversion greatly alters the structural and physicochemical properties of the drug. PFT-alpha has a pKa of 9.11 and is an ionic species in physiological medium, whereas the tricyclic derivative has a pKa of 4.36 and exists as the neutral free base at pH 7. The tricyclic derivative is very hydrophobic, with a log P of 4.26. Although PFT-alpha is generally used at 10-30 microM concentration, the aqueous solubility of its derivative is only 0.2 microM, and it can form a visible precipitate under conditions of typical use. The conversion of PFT-alpha proceeds via an intramolecular cyclization reaction involving the imine and carbonyl groups. Modification of the carbonyl function creates a stable analogue of PFT-alpha that remains soluble indefinitely. These results provide a strategy for the rational design of PFT-alpha analogues that exhibit predictable stability, hydrophobicity, and aqueous solubility.

 网站首页
网站首页