概要
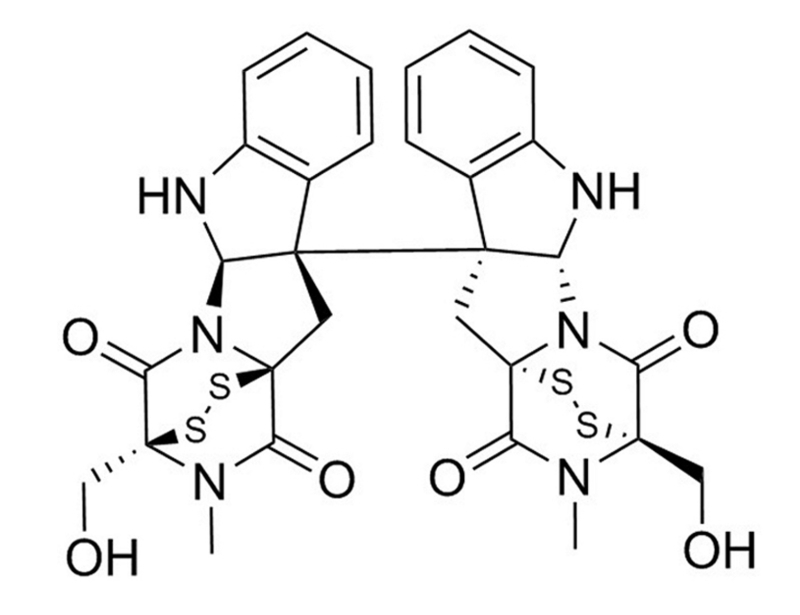
Chaetocin is a fungal mycotoxin, originally produced by Chaetomium species, that inhibits the lysine-specific histone methyltransferases (HMTs) SU(VAR)3-9 (IC₅₀ = 0.8 μM). HMTs are responsible for methylation of histones, thereby affecting heterochromatinization. Chaetocin is also known to inhibit G9a and DIM5 (IC₅₀ = 2.5 μM and 3 μM respectively; Cherblanc et al.; Greiner et al.). Chaetocin also acts as a competitive substrate and inhibitor of the central oxidative stress remediation enzyme thioredoxin reductase-1 (TrxR1; Km = 4.6 μM) more potently than glutathione reductase or thioredoxin (Tibodeau et al.).
CANCER RESEARCH
· Induces cellular oxidative stress, selectively killing cancer cells and rapidly-proliferating primary cells (Isham et al.; Spannhoff et al.)
· Attenuates the growth of glioma xenografts accompanied by an increase in reactive oxygen species (ROS) production (Dixit et al.).
CANCER RESEARCH
· Induces cellular oxidative stress, selectively killing cancer cells and rapidly-proliferating primary cells (Isham et al.; Spannhoff et al.)
· Attenuates the growth of glioma xenografts accompanied by an increase in reactive oxygen species (ROS) production (Dixit et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Chaetocin | 73592 | All | English |
| Safety Data Sheet | Chaetocin | 73592 | All | English |
数据及文献
Publications (6)
Cell death & disease 2014 JAN
Chaetocin-induced ROS-mediated apoptosis involves ATM-YAP1 axis and JNK-dependent inhibition of glucose metabolism.
Abstract
Abstract
Oxidative stress serves as an important regulator of both apoptosis and metabolic reprogramming in tumor cells. Chaetocin, a histone methyltransferase inhibitor, is known to induce ROS generation. As elevating basal ROS level sensitizes glioma cells to apoptosis, the ability of Chaetocin in regulating apoptotic and metabolic adaptive responses in glioma was investigated. Chaetocin induced glioma cell apoptosis in a ROS-dependent manner. Increased intracellular ROS induced (i) Yes-associated protein 1 (YAP1) expression independent of the canonical Hippo pathway as well as (ii) ATM and JNK activation. Increased interaction of YAP1 with p73 and p300 induced apoptosis in an ATM-dependent manner. Chaetocin induced JNK modulated several metabolic parameters like glucose uptake, lactate production, ATP generation, and activity of glycolytic enzymes hexokinase and pyruvate kinase. However, JNK had no effect on ATM or YAP1 expression. Coherent with the in vitro findings, Chaetocin reduced tumor burden in heterotypic xenograft glioma mouse model. Chaetocin-treated tumors exhibited heightened ROS, pATM, YAP1 and pJNK levels. Our study highlights the coordinated control of glioma cell proliferation and metabolism by ROS through (i) ATM-YAP1-driven apoptotic pathway and (ii) JNK-regulated metabolic adaptation. The elucidation of these newfound connections and the roles played by ROS to simultaneously shift metabolic program and induce apoptosis could provide insights toward the development of new anti-glioma strategies.
Nature chemical biology 2013 MAR
Chaetocin is a nonspecific inhibitor of histone lysine methyltransferases.
Abstract
Abstract
Antioxidants & redox signaling 2009 MAY
The anticancer agent chaetocin is a competitive substrate and inhibitor of thioredoxin reductase.
Abstract
Abstract
We recently reported that the antineoplastic thiodioxopiperazine natural product chaetocin potently induces cellular oxidative stress, thus selectively killing cancer cells. In pursuit of underlying molecular mechanisms, we now report that chaetocin is a competitive and selective substrate for the oxidative stress mitigation enzyme thioredoxin reductase-1 (TrxR1) with lower K(m) than the TrxR1 native substrate thioredoxin (Trx; chaetocin K(m) = 4.6 +/- 0.6 microM, Trx K(m) = 104.7 +/- 26 microM), thereby attenuating reduction of the critical downstream ROS remediation substrate Trx at achieved intracellular concentrations. Consistent with a role for TrxR1 targeting in the anticancer effects of chaetocin, overexpression of the TrxR1 downstream effector Trx in HeLa cells conferred resistance to chaetocin-induced, but not to doxorubicin-induced, cytotoxicity. As the TrxR/Trx pathway is of central importance in limiting cellular reactive oxygen species (ROS)--and as chaetocin exerts its selective anticancer effects via ROS imposition--the inhibition of TrxR1 by chaetocin has potential to explain its selective anticancer effects. These observations have important implications not just with regard to the mechanism of action and clinical development of chaetocin and related thiodioxopiperazines, but also with regard to the utility of molecular targets within the thioredoxin reductase/thioredoxin pathway in the development of novel candidate antineoplastic agents.
The international journal of biochemistry & cell biology 2009 JAN
Cancer treatment of the future: inhibitors of histone methyltransferases.
Abstract
Abstract
Cancer in humans is the result of a multi-step process. This process often involves the activation of oncogenes and/or the inactivation of tumor suppressor genes. These two steps arise not only due to mutations, but can also be the result of a translocation or an altered transcription rate. One important mechanism is the occurrence of epigenetic alterations like promotor methylation (which may lead to tumor suppressor silencing) or decreased histone acetylation (which can result in the downregulation of proteins involved in apoptosis). Today, histone acetylation and DNA methylation are epigenetic modifications which have been linked closely to the pathology of human cancers and inhibitors of both enzyme classes for clinical use are at hand. In contrast, other fields of epigenetics still lack of similarly thorough knowledge. This is especially true for the group of histone methyltransferases and their inhibitors. Since connections between histone methylation patterns and cancer progression have been recognized, histone methyltransferases represent promising targets for future cancer treatment.
Blood 2007 MAR
Chaetocin: a promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress.
Abstract
Abstract
Chaetocin, a thiodioxopiperazine natural product previously unreported to have anticancer effects, was found to have potent antimyeloma activity in IL-6-dependent and -independent myeloma cell lines in freshly collected sorted and unsorted patient CD138(+) myeloma cells and in vivo. Chaetocin largely spares matched normal CD138(-) patient bone marrow leukocytes, normal B cells, and neoplastic B-CLL (chronic lymphocytic leukemia) cells, indicating a high degree of selectivity even in closely lineage-related B cells. Furthermore, chaetocin displays superior ex vivo antimyeloma activity and selectivity than doxorubicin and dexamethasone, and dexamethasone- or doxorubicin-resistant myeloma cell lines are largely non-cross-resistant to chaetocin. Mechanistically, chaetocin is dramatically accumulated in cancer cells via a process inhibited by glutathione and requiring intact/unreduced disulfides for uptake. Once inside the cell, its anticancer activity appears mediated primarily through the imposition of oxidative stress and consequent apoptosis induction. Moreover, the selective antimyeloma effects of chaetocin appear not to reflect differential intracellular accumulation of chaetocin but, instead, heightened sensitivity of myeloma cells to the cytotoxic effects of imposed oxidative stress. Considered collectively, chaetocin appears to represent a promising agent for further study as a potential antimyeloma therapeutic.
Nature chemical biology 2005 AUG
Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9.
Abstract
Abstract
Histone methylation plays a key role in establishing and maintaining stable gene expression patterns during cellular differentiation and embryonic development. Here, we report the characterization of the fungal metabolite chaetocin as the first inhibitor of a lysine-specific histone methyltransferase. Chaetocin is specific for the methyltransferase SU(VAR)3-9 both in vitro and in vivo and may therefore be used to study heterochromatin-mediated gene repression.

 网站首页
网站首页