概要
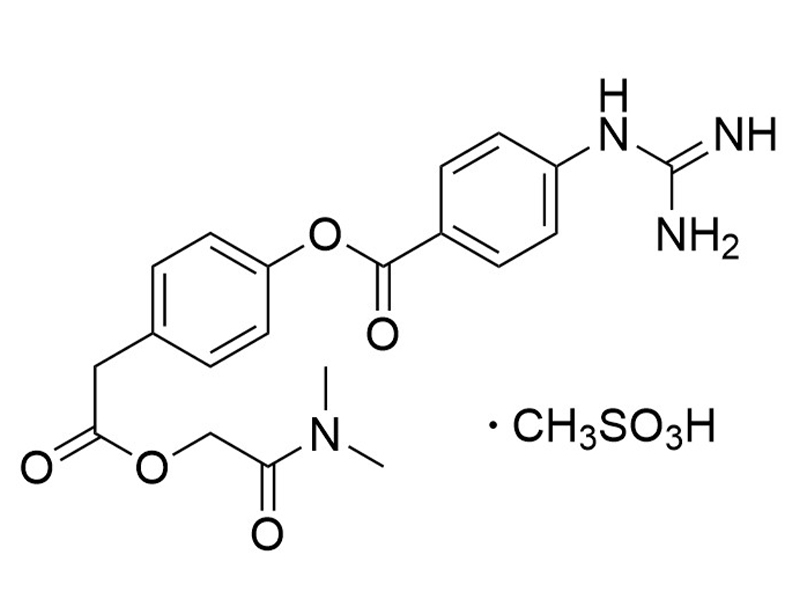
Camostat is a serine protease inhibitor with antiviral properties (Coote et al.; Hoffmann et al.). It inhibits the transmembrane serine protease TMPRSS2, which is involved in viral infections such as COVID-19 (Hoffmann et al.). This product is supplied as the mesylate salt of the molecule.
IMMUNOLOGY
· Inhibits MCP-1 and TNF-alpha production in monocytes (Gibo et al.).
DISEASE MODELING
· Inhibits the proliferation and activation of pancreatic stellate cells in rats (Emori et al.).
IMMUNOLOGY
· Inhibits MCP-1 and TNF-alpha production in monocytes (Gibo et al.).
DISEASE MODELING
· Inhibits the proliferation and activation of pancreatic stellate cells in rats (Emori et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Camostat (Mesylate) | 100-0552, 100-0553 | All | English |
| Safety Data Sheet | Camostat (Mesylate) | 100-0552, 100-0553 | All | English |
数据及文献
Publications (4)
Cell 2020
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor.
Abstract
Abstract
The recent emergence of the novel, pathogenic SARS-coronavirus 2 (SARS-CoV-2) in China and its rapid national and international spread pose a global health emergency. Cell entry of coronaviruses depends on binding of the viral spike (S) proteins to cellular receptors and on S protein priming by host cell proteases. Unravelling which cellular factors are used by SARS-CoV-2 for entry might provide insights into viral transmission and reveal therapeutic targets. Here, we demonstrate that SARS-CoV-2 uses the SARS-CoV receptor ACE2 for entry and the serine protease TMPRSS2 for S protein priming. A TMPRSS2 inhibitor approved for clinical use blocked entry and might constitute a treatment option. Finally, we show that the sera from convalescent SARS patients cross-neutralized SARS-2-S-driven entry. Our results reveal important commonalities between SARS-CoV-2 and SARS-CoV infection and identify a potential target for antiviral intervention.
The Journal of pharmacology and experimental therapeutics 2009 may
Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease.
Abstract
Abstract
Inhibition of airway epithelial sodium channel (ENaC) function enhances mucociliary clearance (MCC). ENaC is positively regulated by channel-activating proteases (CAPs), and CAP inhibitors are therefore predicted to be beneficial in diseases associated with impaired MCC. The aims of the present study were to 1) identify low-molecular-weight inhibitors of airway CAPs and 2) to establish whether such CAP inhibitors would translate into a negative regulation of ENaC function in vivo, with a consequent enhancement of MCC. To this end, camostat, a trypsin-like protease inhibitor, provided a potent (IC(50) approximately 50 nM) and prolonged attenuation of ENaC function in human airway epithelial cell models that was reversible upon the addition of excess trypsin. In primary human bronchial epithelial cells, a potency order of placental bikunin {\textgreater} camostat {\textgreater} 4-guanidinobenzoic acid 4-carboxymethyl-phenyl ester {\textgreater} aprotinin {\textgreater} soybean trypsin inhibitor = alpha1-antitrypsin, was largely consistent with that observed for inhibition of prostasin, a molecular candidate for the airway CAP. In vivo, topical airway administration of camostat induced a potent and prolonged attenuation of ENaC activity in the guinea pig trachea (ED(50) = 3 microg/kg). When administered by aerosol inhalation in conscious sheep, camostat enhanced MCC out to at least 5 h after inhaled dosing. In summary, camostat attenuates ENaC function and enhances MCC, providing an opportunity for this approach toward the negative regulation of ENaC function to be tested therapeutically.
Journal of gastroenterology and hepatology 2005 jun
Camostat, an oral trypsin inhibitor, reduces pancreatic fibrosis induced by repeated administration of a superoxide dismutase inhibitor in rats.
Abstract
Abstract
BACKGROUND AND AIM An oral trypsin inhibitor, camostat (CM), has a beneficial effect on chronic pancreatitis, but its mechanism is not yet fully understood. Recently, pancreatic stellate cells (PSC) have been reported to play an essential role in pancreatic fibrosis. An experimental model of pancreatic fibrosis induced by a superoxide dismutase (SOD) inhibitor (diethyldithiocarbamate [DDC]) was developed in rats. Thus, the effect of an oral trypsin inhibitor on pancreatic fibrosis and PSC was investigated. METHODS Pancreatic fibrosis was induced in rats using DDC (DDC rats). DDC + CM rats were administered DDC, and subsequently were fed a diet containing CM. Immunohistochemistry of the pancreas was performed with monoclonal anti-alpha-smooth muscle actin (alpha-SMA) antibody and anti-desmin antibody. RESULTS The DDC rats showed a significant increase in alpha-SMA-positive cells or desmin-positive cells compared with control rats. These significant increases in the fibrotic area improved after treatment with CM. The level of prolyl hydroxylase in the pancreas, which significantly increased as a result of DDC, decreased after treatment with CM. CONCLUSION Camostat has a beneficial effect on pancreatic fibrosis induced by the administration of a SOD inhibitor, which inhibits the proliferation and activation of PSC.
Laboratory investigation; a journal of technical methods and pathology 2005 jan
Camostat mesilate attenuates pancreatic fibrosis via inhibition of monocytes and pancreatic stellate cells activity.
Abstract
Abstract
Camostat mesilate (CM), an oral protease inhibitor, has been used clinically for the treatment of chronic pancreatitis in Japan. However, the mechanism by which it operates has not been fully understood. Our aim was to evaluate the therapeutic efficacy of CM in the experimental pancreatic fibrosis model induced by dibutyltin dichloride (DBTC), and we also determined the effect of CM on isolated monocytes and panceatic stellate cells (PSCs). In vivo, chronic pancreatitis was induced in male Lewis rats by single administration of 7 mg/kg DBTC and a special diet containing 1 mg/g CM was fed to the DBTC+CM-treated group from day 7, while the DBTC-treated group rats were fed a standard diet. At days 0, 7, 14 and 28, the severity of pancreatitis and fibrosis was examined histologically and enzymologically in both groups. In vitro, monocytes were isolated from the spleen of a Lewis rat, and activated with lipopolysaccharide stimulation. Thereafter, the effect of CM on monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-alpha (TNF-alpha) production from monocytes was examined. Subsequently, cultured rat PSCs were exposed to CM and tested to see whether their proliferation, MCP-1 production and procollagen alpha1 messenger RNA expression was influenced by CM. In vivo, the oral administration of CM inhibited inflammation, cytokines expression and fibrosis in the pancreas. The in vitro study revealed that CM inhibited both MCP-1 and TNF-alpha production from monocytes, and proliferation and MCP-1 production from PSCs. However, procollagen alpha1 expression in PSCs was not influenced by CM. These results suggest that CM attenuated DBTC-induced rat pancreatic fibrosis via inhibition of monocytes and PSCs activity.

 网站首页
网站首页