概要
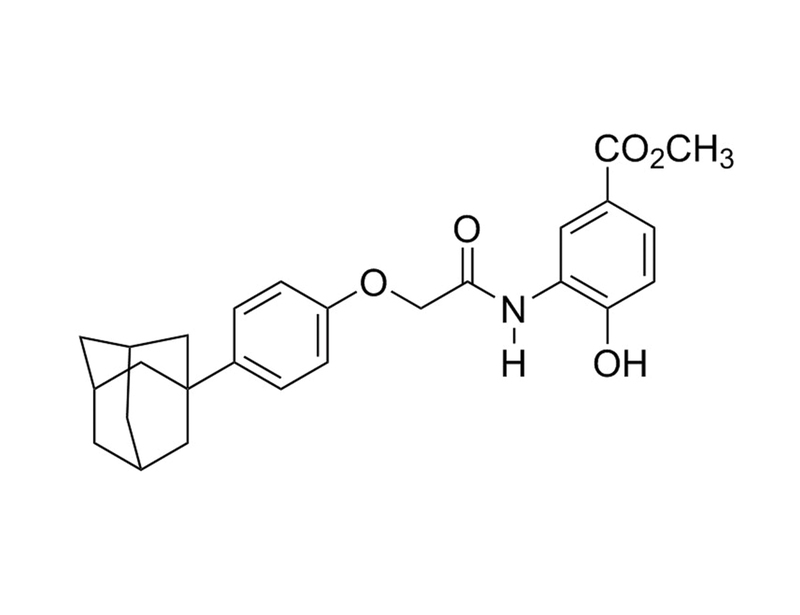
CAY10585 inhibits hypoxia inducible factor 1 (HIF-1) accumulation in a concentration and time-dependent manner and significantly suppresses transcriptional activity of HIF-1 target genes (Lee et al.).
DIFFERENTIATION
· Inhibits BMP9- and HIF-1-induced osteogenic differentiation in mesenchymal stem cells (Hu et al.).
· Rescues cardiomyocyte differentiation in Fgfr1(-/-) mouse embryonic stem cells (Crescini et al.).
DIFFERENTIATION
· Inhibits BMP9- and HIF-1-induced osteogenic differentiation in mesenchymal stem cells (Hu et al.).
· Rescues cardiomyocyte differentiation in Fgfr1(-/-) mouse embryonic stem cells (Crescini et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | CAY10585 | 72432 | All | English |
| Safety Data Sheet | CAY10585 | 72432 | All | English |
数据及文献
Publications (5)
Biochimica et biophysica acta 2013 JAN
Ascorbic acid rescues cardiomyocyte development in Fgfr1(-/-) murine embryonic stem cells.
Abstract
Abstract
Fibroblast growth factor receptor 1 (Fgfr1) gene knockout impairs cardiomyocyte differentiation in murine embryonic stem cells (mESC). Here, various chemical compounds able to enhance cardiomyocyte differentiation in mESC [including dimethylsulfoxide, ascorbic acid (vitC), free radicals and reactive oxygen species] were tested for their ability to rescue the cardiomyogenic potential of Fgfr1(-/-) mESC. Among them, only the reduced form of vitC, l-ascorbic acid, was able to recover beating cell differentiation in Fgfr1(-/-) mESC. The appearance of contracting cells was paralleled by the expression of early and late cardiac gene markers, thus suggesting their identity as cardiomyocytes. In the attempt to elucidate the mechanism of action of vitC on Fgfr1(-/-) mESC, we analyzed several parameters related to the intracellular redox state, such as reactive oxygen species content, Nox4 expression, and superoxide dismutase activity. The results did not show any relationship between the antioxidant capacity of vitC and cardiomyocyte differentiation in Fgfr1(-/-) mESC. No correlation was found also for the ability of vitC to modulate the expression of pluripotency genes. Then, we tested the hypothesis that vitC was acting as a prolyl hydroxylase cofactor by maintaining iron in a reduced state. We first analyze hypoxia inducible factor (HIF)-1α mRNA and protein levels that were found to be slightly upregulated in Fgfr1(-/-) cells. We treated mESC with Fe(2+) or the HIF inhibitor CAY10585 during the first phases of the differentiation process and, similar to vitC, the two compounds were able to rescue cardiomyocyte formation in Fgfr1(-/-) mESC, thus implicating HIF-1α modulation in Fgfr1-dependent cardiomyogenesis.
Journal of cell science 2013 JAN
BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells.
Abstract
Abstract
Mesenchymal stromal progenitor cells (MSCs) are multipotent progenitors that can be isolated from numerous tissues. MSCs can undergo osteogenic differentiation under proper stimuli. We have recently demonstrated that bone morphogenetic protein 9 (BMP9) is one of the most osteogenic BMPs. As one of the least studied BMPs, BMP9 has been shown to regulate angiogenesis in endothelial cells. However, it is unclear whether BMP9-regulated angiogenic signaling plays any important role in the BMP9-initiated osteogenic pathway in MSCs. Here, we investigate the functional role of hypoxia-inducible factor 1α (HIF1α)-mediated angiogenic signaling in BMP9-regulated osteogenic differentiation of MSCs. We find that BMP9 induces HIF1α expression in MSCs through Smad1/5/8 signaling. Exogenous expression of HIF1α potentiates BMP9-induced osteogenic differentiation of MSCs both in vitro and in vivo. siRNA-mediated silencing of HIF1α or HIF1α inhibitor CAY10585 profoundly blunts BMP9-induced osteogenic signaling in MSCs. HIF1α expression regulated by cobalt-induced hypoxia also recapitulates the synergistic effect between HIF1α and BMP9 in osteogenic differentiation. Mechanistically, HIF1α is shown to exert its synergistic effect with BMP9 by inducing both angiogenic signaling and osteogenic signaling in MSCs. Thus, our findings should not only expand our understanding of the molecular basis behind BMP9-regulated osteoblastic lineage-specific differentiation, but also provide an opportunity to harness the BMP9-induced synergy between osteogenic and angiogenic signaling pathways in regenerative medicine.
Journal of medicinal chemistry 2007 APR
(Aryloxyacetylamino)benzoic acid analogues: A new class of hypoxia-inducible factor-1 inhibitors.
Abstract
Abstract
Structural modification of a compound discovered during screening using an HRE-dependent reporter assay has revealed a novel class of HIF-1 inhibitors, which potently inhibit the HIF-1alpha protein accumulation and its target gene expression under hypoxic conditions in human hepatocellular carcinoma Hep3B cells.
The Journal of clinical investigation 2003 MAR
HIF hydroxylation and the mammalian oxygen-sensing pathway.
Abstract
Abstract
Proceedings of the National Academy of Sciences of the United States of America 1995 JUN
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension.
Abstract
Abstract
Hypoxia-inducible factor 1 (HIF-1) is found in mammalian cells cultured under reduced O2 tension and is necessary for transcriptional activation mediated by the erythropoietin gene enhancer in hypoxic cells. We show that both HIF-1 subunits are basic-helix-loop-helix proteins containing a PAS domain, defined by its presence in the Drosophila Per and Sim proteins and in the mammalian ARNT and AHR proteins. HIF-1 alpha is most closely related to Sim. HIF-1 beta is a series of ARNT gene products, which can thus heterodimerize with either HIF-1 alpha or AHR. HIF-1 alpha and HIF-1 beta (ARNT) RNA and protein levels were induced in cells exposed to 1% O2 and decayed rapidly upon return of the cells to 20% O2, consistent with the role of HIF-1 as a mediator of transcriptional responses to hypoxia.

 网站首页
网站首页