概要
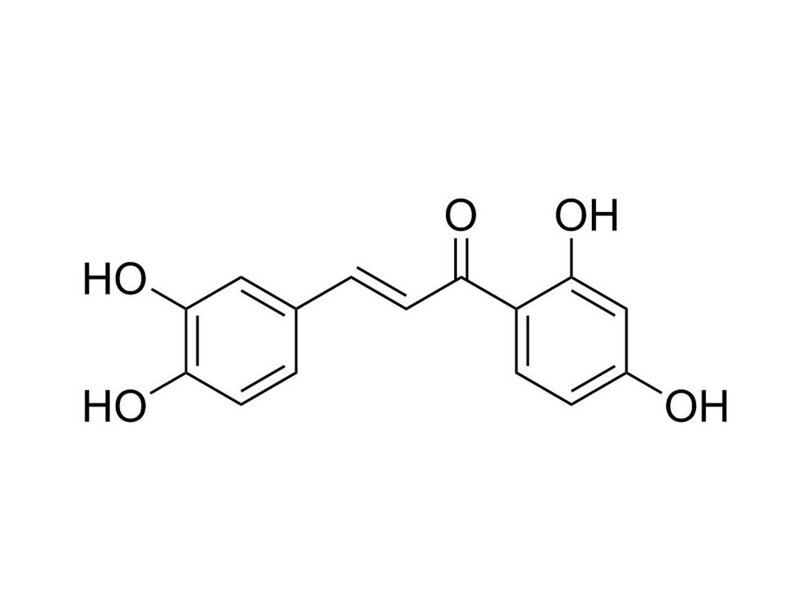
Butein is a plant polyphenol, tetrahydroxychalcone that inhibits nuclear factor (NF)-κB (Yang et al; Pandey et al. 2007). Butein has been shown to prevent phosphorylation and degradation of TNF-dependent IκBα, an inhibitory subunit of NF-κB (IC₅₀ = 38 μM; Orlikova et al.). Butein also inhibits 5-lipoxygenase (IC₅₀ = 0.01 μM; Sogawa et al.), the enoyl-acyl-carrier protein reductase of Plasmodium falciparum (Ki = 2.97 μM; Sharma et al.), angiotensin-converting enzyme (IC₅₀ = 0.73 mM; Bonesi et al.), and SRC kinase (Pandey et al. 2009).
METABOLISM
· Inhibits iron-induced lipid peroxidation in rat brain homogenate in a concentration-dependent manner (Cheng et al.).
CANCER RESEARCH
· Inhibits TNF-α-induced invasion of human lung adenocarcinoma H1299 cells (Pandey et al. 2007; Gupta et al.).
· Inhibits growth in human hepatoma cancer cell lines HepG2 and Hep3B, by inducing G2/M phase arrest (Moon et al.; Gupta et al.).
· Inhibits testosterone-induced proliferation in breast cancer cells (Wang et al.)
IMMUNOLOGY
· Exhibits anti-inflammatory properties in a mouse macrophage cell line by inhibiting LPS-induced expression of iNOS (Lee et al.).
METABOLISM
· Inhibits iron-induced lipid peroxidation in rat brain homogenate in a concentration-dependent manner (Cheng et al.).
CANCER RESEARCH
· Inhibits TNF-α-induced invasion of human lung adenocarcinoma H1299 cells (Pandey et al. 2007; Gupta et al.).
· Inhibits growth in human hepatoma cancer cell lines HepG2 and Hep3B, by inducing G2/M phase arrest (Moon et al.; Gupta et al.).
· Inhibits testosterone-induced proliferation in breast cancer cells (Wang et al.)
IMMUNOLOGY
· Exhibits anti-inflammatory properties in a mouse macrophage cell line by inhibiting LPS-induced expression of iNOS (Lee et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Butein | 73462, 73464 | All | English |
| Safety Data Sheet | Butein | 73462, 73464 | All | English |
数据及文献
Publications (12)
Oncology reports 2012 SEP
Natural chalcones as dual inhibitors of HDACs and NF-κB.
Abstract
Abstract
Histone deacetylase enzymes (HDACs) are emerging as a promising biological target for cancer and inflammation. Using a fluorescence assay, we tested the in vitro HDAC inhibitory activity of twenty-one natural chalcones, a widespread group of natural products with well-known anti-inflammatory and antitumor effects. Since HDACs regulate the expression of the transcription factor NF-κB, we also evaluated the inhibitory potential of the compounds on NF-κB activation. Only four chalcones, isoliquiritigenin (no. 10), butein (no. 12), homobutein (no. 15) and the glycoside marein (no. 21) showed HDAC inhibitory activity with IC50 values of 60-190 µM, whereas a number of compounds inhibited TNFα-induced NF-κB activation with IC50 values in the range of 8-41 µM. Interestingly, three chalcones (nos. 10, 12 and 15) inhibited both TNFα-induced NF-κB activity and total HDAC activity of classes I, II and IV. Molecular modeling and docking studies were performed to shed light into dual activity and to draw structure-activity relationships among chalcones (nos. 1-21). To the best of our knowledge this is the first study that provides evidence for HDACs as potential drug targets for natural chalcones. The dual inhibitory potential of the selected chalcones on NF-κB and HDACs was investigated for the first time. This study demonstrates that chalcones can serve as lead compounds in the development of dual inhibitors against both targets in the treatment of inflammation and cancer.
Cancer metastasis reviews 2010 SEP
Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals.
Abstract
Abstract
Almost 25 centuries ago, Hippocrates, the father of medicine, proclaimed Let food be thy medicine and medicine be thy food." Exploring the association between diet and health continues today. For example�
Bioorganic & medicinal chemistry letters 2010 MAR
The synthesis and angiotensin converting enzyme (ACE) inhibitory activity of chalcones and their pyrazole derivatives.
Abstract
Abstract
A series of chalcones (1-9) and pyrazoles (10-18) was prepared to investigate their potential activity as Angiotensin I-Converting Enzyme (ACE) inhibitors. Their structures were verified by elemental analysis, UV, IR, MS, (1)H NMR, (13)C NMR, and 2D NMR experiments. Among tested compounds, chalcone 7 exerted the highest activity with an IC(50) value of 0.219 mM, while the most potent pyrazole was 15 (IC(50) value of 0.213 mM).
Cancer letters 2010 FEB
Butein induces G(2)/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation.
Abstract
Abstract
We investigated the molecular effects of 3,4,2',4'-tetrahydroxychalcone (butein) treatment in two human hepatoma cancer cell lines-HepG2 and Hep3B. Butein treatment inhibited cancer cell growth by inducing G(2)/M phase arrest and apoptosis. Butein-induced G(2)/M phase arrest was associated with increased ATM, Chk1, and Chk2 phosphorylations and reduced cdc25C levels. Additionally, butein treatment enhanced inactivated phospho-Cdc2 levels, reduced Cdc2 kinase activity, and generated reactive oxygen species (ROS) that was accompanied by JNK activation. The extent of butein-induced G(2)/M phase arrest significantly decreased following pretreatment with N-acetyl-l-cysteine or glutathione and following JNK phosphorylation reduction by SP600125. Both N-acetyl-l-cysteine and glutathione also decreased butein-mediated apoptosis. Taken together, these results imply a critical role of ROS and JNK in the anticancer effects of butein.
Molecular pharmacology 2009 MAR
Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1.
Abstract
Abstract
The aim of the current study is to determine whether butein (3,4,2',4'-tetrahydroxychalcone) exhibits antiproliferative effects against tumor cells through suppression of the signal transducer and activator of transcription 3 (STAT3) activation pathway. We investigated the effects of butein on constitutive and inducible STAT3 activation, role of tyrosine kinases and phosphatases in STAT3 activation, STAT3-regulated gene products, and growth modulation of tumor cells. We found that this chalcone inhibited both constitutive and interleukin-6-inducible STAT3 activation in multiple myeloma (MM) cells. The suppression was mediated through the inhibition of activation of the upstream kinases c-Src, Janus-like kinase (JAK) 1, and JAK2. Vanadate treatment reversed the butein-induced down-regulation of STAT3 activation, suggesting the involvement of a tyrosine phosphatase. Indeed, we found that butein induced the expression of the tyrosine phosphatase SHP-1 and deletion of SHP-1 gene by small interfering RNA abolished the ability of butein to inhibit STAT3 activation, suggesting the critical role of SHP-1 in the action of this chalcone. Butein down-regulated the expression of STAT3-regulated gene products such as Bcl-xL, Bcl-2, cyclin D1, and Mcl-1, and this led to the suppression of proliferation and induction of apoptosis. Consistent with these results, overexpression of constitutive active STAT3 significantly reduced the butein-induced apoptosis. Moreover, we found that butein significantly potentiated the apoptotic effects of thalidomide and Velcade in MM cells. Overall, these results suggest that butein is a novel blocker of STAT3 activation and thus may have potential in suppression of tumor cell proliferation and reversal of chemoresistance in MM cells.
The Journal of biological chemistry 2007 JUN
Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue.
Abstract
Abstract
Although butein (3,4,2',4'-tetrahydroxychalcone) is known to exhibit anti-inflammatory, anti-cancer, and anti-fibrogenic activities, very little is known about its mechanism of action. Because numerous effects modulated by butein can be linked to interference with the NF-kappaB pathway, we investigated in detail the effect of this chalcone on NF-kappaB activity. As examined by DNA binding, we found that butein suppressed tumor necrosis factor (TNF)-induced NF-kappaB activation in a dose- and time-dependent manner; suppressed the NF-kappaB activation induced by various inflammatory agents and carcinogens; and inhibited the NF-kappaB reporter activity induced by TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, and IKK-beta. We also found that butein blocked the phosphorylation and degradation of IkappaBalpha by inhibiting IkappaBalpha kinase (IKK) activation. We found the inactivation of IKK by butein was direct and involved cysteine residue 179. This correlated with the suppression of phosphorylation and the nuclear translocation of p65. In this study, butein also inhibited the expression of the NF-kappaB-regulated gene products involved in anti-apoptosis (IAP2, Bcl-2, and Bcl-xL), proliferation (cyclin D1 and c-Myc), and invasion (COX-2 and MMP-9). Suppression of these gene products correlated with enhancement of the apoptosis induced by TNF and chemotherapeutic agents; and inhibition of cytokine-induced cellular invasion. Overall, our results indicated that antitumor and anti-inflammatory activities previously assigned to butein may be mediated in part through the direct inhibition of IKK, leading to the suppression of the NF-kappaB activation pathway.

 网站首页
网站首页