概要
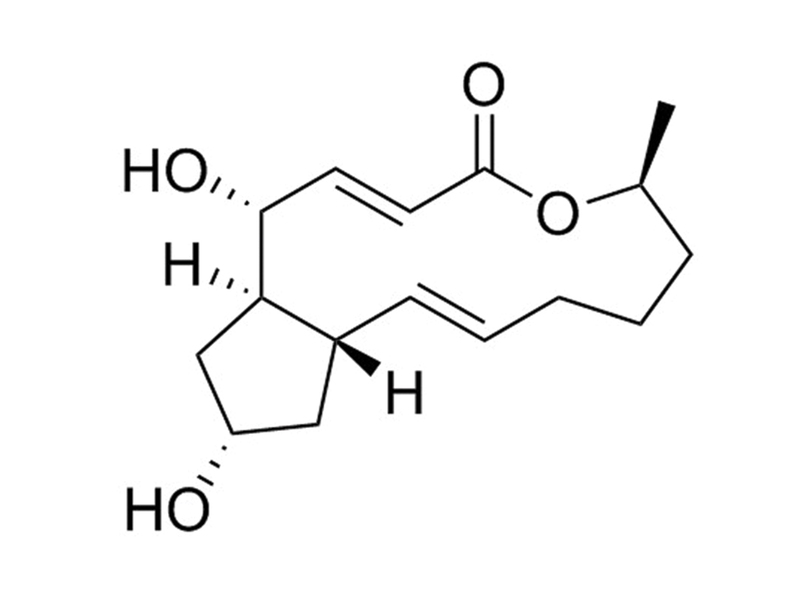
Brefeldin A is a fungal lactone antibiotic, produced by many species, including Eupenicillium brefeldianum (Klausner et al.). It reversibly interferes with protein trafficking and secretion mediated by the Golgi apparatus and endoplasmic reticulum by indirect inhibition of ADP-ribosylation factor (ARF; Klausner et al.; Helms & Rothman; Robinson et al.; Morinaga et al.; Moss & Vaughan; Nebenführ et al.; Ktistakis et al.). Brefeldin A binds to Sec7-containing guanine-exchange factor (GEF) at the ARF-GDP-Sec7 interface, preventing the conformational change required to release GDP and activate ARF (Mossessova et al.).
CELL LINE DEVELOPMENT
· Improves clustered regularly interspaced palindromic repeats (CRISPR)-mediated homology-directed repair (HDR) in mouse embryonic stem cells (Yu et al.).
CANCER RESEARCH
· Induces apoptosis in human leukemia (HL60, K562) and colon carcinoma (HT-29) cell lines (Shao et al.).
· Reduces survival, induces apoptosis and inhibits clonogenic activity of Colo 205 colorectal cancer stem cell line (Tseng et al.).
CELL LINE DEVELOPMENT
· Improves clustered regularly interspaced palindromic repeats (CRISPR)-mediated homology-directed repair (HDR) in mouse embryonic stem cells (Yu et al.).
CANCER RESEARCH
· Induces apoptosis in human leukemia (HL60, K562) and colon carcinoma (HT-29) cell lines (Shao et al.).
· Reduces survival, induces apoptosis and inhibits clonogenic activity of Colo 205 colorectal cancer stem cell line (Tseng et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Brefeldin A | 73012, 73014 | All | English |
| Safety Data Sheet | Brefeldin A | 73012, 73014 | All | English |
数据及文献
Publications (11)
Cell stem cell 2015 FEB
Small molecules enhance CRISPR genome editing in pluripotent stem cells.
Abstract
Abstract
The bacterial CRISPR-Cas9 system has emerged as an effective tool for sequence-specific gene knockout through non-homologous end joining (NHEJ), but it remains inefficient for precise editing of genome sequences. Here we develop a reporter-based screening approach for high-throughput identification of chemical compounds that can modulate precise genome editing through homology-directed repair (HDR). Using our screening method, we have identified small molecules that can enhance CRISPR-mediated HDR efficiency, 3-fold for large fragment insertions and 9-fold for point mutations. Interestingly, we have also observed that a small molecule that inhibits HDR can enhance frame shift insertion and deletion (indel) mutations mediated by NHEJ. The identified small molecules function robustly in diverse cell types with minimal toxicity. The use of small molecules provides a simple and effective strategy to enhance precise genome engineering applications and facilitates the study of DNA repair mechanisms in mammalian cells.
Molecules (Basel, Switzerland) 2013
Brefeldin a effectively inhibits cancer stem cell-like properties and MMP-9 activity in human colorectal cancer Colo 205 cells.
Abstract
Abstract
Cancer stem cells (CSCs) are a small subset of cancer cells with indefinite potential for self-renewal and the capacity to drive tumorigenesis. Brefeldin A (BFA) is an antibiotic that is known to block protein transport and induce endoplasmic reticulum (ER) stress in eukaryotic cells, but its effects on colorectal CSCs are unknown. We investigated the inhibitory effect of BFA on human colorectal cancer Colo 205 cells. We found that BFA effectively reduced the survival of suspension Colo 205 cells (IC₅₀ = ˜15 ng/mL) by inducing apoptosis, and inhibited the clonogenic activity of Colo 205 CSCs in tumorsphere formation assay and soft agar colony formation assay in the same nanogram per milliliter range. We also discovered that at such low concentrations, BFA effectively induced endoplasmic reticulum (ER) stress response as indicated by the increased mRNA expression of ER stress-related genes, such as glucose-regulated protein 78 (GRP78), X-box binding protein 1 (XBP1), and C/EBP homologous protein (CHOP). Finally, we found that BFA reduced the activity of matrix metallopeptidase 9 (MMP-9). These findings suggest that BFA can effectively suppress the progression of colorectal cancer during the tumorigenesis and metastasis stages. These results may lead to the development of novel therapies for the treatment of colorectal cancer.
Plant physiology 2008 AUG
The endosomal system of plants: charting new and familiar territories.
Abstract
Abstract
Molecular cell 2003
Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism.
Abstract
Abstract
ARF GTPases are activated by guanine nucleotide exchange factors (GEFs) of the Sec7 family that promote the exchange of GDP for GTP. Brefeldin A (BFA) is a fungal metabolite that binds to the ARF1*GDP*Sec7 complex and blocks GEF activity at an early stage of the reaction, prior to guanine nucleotide release. The crystal structure of the ARF1*GDP*Sec7*BFA complex shows that BFA binds at the protein-protein interface to inhibit conformational changes in ARF1 required for Sec7 to dislodge the GDP molecule. Based on a comparative analysis of the inhibited complex, nucleotide-free ARF1*Sec7 and ARF1*GDP, we suggest that, in addition to forcing nucleotide release, the ARF1-Sec7 binding energy is used to open a cavity on ARF1 to facilitate the rearrangement of hydrophobic core residues between the GDP and GTP conformations. Thus, the Sec7 domain may act as a dual catalyst, facilitating both nucleotide release and conformational switching on ARF proteins.
Plant physiology 2002
Brefeldin A: deciphering an enigmatic inhibitor of secretion.
Abstract
Abstract
Molecular and cellular biochemistry 1999
Activation of toxin ADP-ribosyltransferases by eukaryotic ADP-ribosylation factors.
Abstract
Abstract
ADP-ribosylation factors (ARFs) are members of a multigene family of 20-kDa guanine nucleotide-binding proteins that are regulatory components in several pathways of intracellular vesicular trafficking. The relatively small (approximately 180-amino acids) ARF proteins interact with a variety of molecules (in addition to GTP/GDP, of course). Cholera toxin was the first to be recognized, hence the name. Later it was shown that ARF also activates phospholipase D. Different parts of the molecule are responsible for activation of the two enzymes. In vesicular trafficking, ARF must interact with coatomer to recruit it to a membrane and thereby initiate vesicle budding. ARF function requires that it alternate between GTP- and GDP-bound forms, which involves interaction with regulatory proteins. Inactivation of ARF-GTP depends on a GTPase-activating protein or GAP. A guanine nucleotide-exchange protein or GEP accelerates release of bound GDP from inactive ARF-GDP to permit GTP binding. Inhibition of GEP by brefeldin A (BFA) blocks ARF activation and thereby vesicular transport. In cells, it causes apparent disintegration of Golgi structure. Both BFA-sensitive and insensitive GEPs are known. Sequences of peptides from a BFA-sensitive GEP purified in our laboratory revealed the presence of a Sec7 domain, a sequence of approximately 200 amino acids that resembles a region in the yeast Sec7 gene product, which is involved in Golgi vesicular transport. Other proteins of unknown function also contain Sec7 domains, among them a lymphocyte protein called cytohesin-1. To determine whether it had GEP activity, recombinant cytohesin-1 was synthesized in E. coli. It preferentially activated class I ARFs 1 and 3 and was not inhibited by BFA but failed to activate ARF5 (class II). There are now five Sec7 domain proteins known to have GEP activity toward class I ARFs. It remains to be determined whether there are other Sec7 domain proteins that are GEPs for ARFs 4, 5, or 6.

 网站首页
网站首页