概要
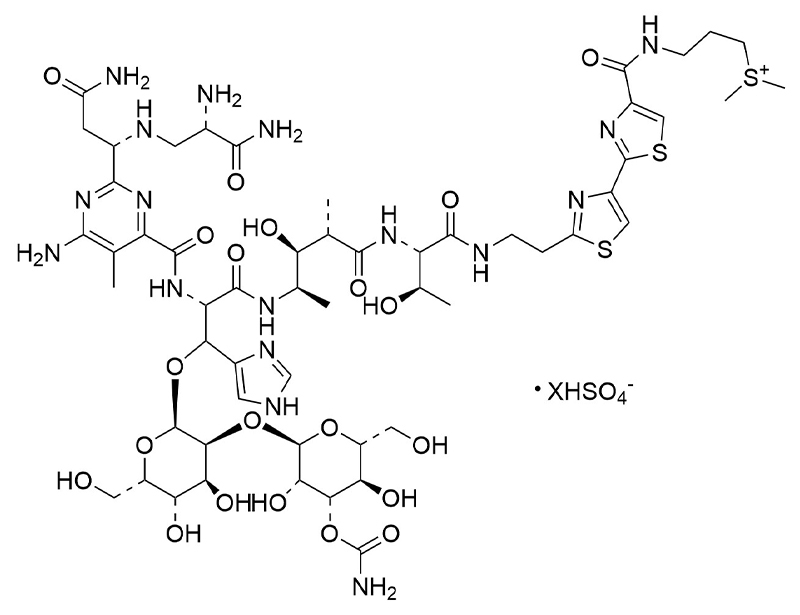
Bleomycin is a glycopeptide-derived antibiotic, isolated from the bacterium Streptomyces verticillus, that creates DNA strand scission and exhibits antitumor activity against squamous cell carcinomas and malignant lymphomas (Galm et al.). Bleomycin induces lung injury leading to acute inflammatory response and pulmonary fibrosis (Kulkarni et al.). This product is supplied as the sulfate salt of the molecule.
CANCER RESEARCH
· Induces single- and double-strand DNA breaks in tumor cells (Hecht).
DISEASE MODELING
· Induces lung fibrosis in mice (Kulkarni et al.).
CANCER RESEARCH
· Induces single- and double-strand DNA breaks in tumor cells (Hecht).
DISEASE MODELING
· Induces lung fibrosis in mice (Kulkarni et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Bleomycin (Sulfate) | 100-0550, 100-0551 | All | English |
| Safety Data Sheet | Bleomycin (Sulfate) | 100-0550, 100-0551 | All | English |
数据及文献
Publications (3)
PloS one 2013
The triterpenoid CDDO-Me inhibits bleomycin-induced lung inflammation and fibrosis.
Abstract
Abstract
Pulmonary Fibrosis (PF) is a devastating progressive disease in which normal lung structure and function is compromised by scarring. Lung fibrosis can be caused by thoracic radiation, injury from chemotherapy and systemic diseases such as rheumatoid arthritis that involve inflammatory responses. CDDO-Me (Methyl 2-cyano-3,12-dioxooleana-1,9(11)dien-28-oate, Bardoxolone methyl) is a novel triterpenoid with anti-fibrotic and anti-inflammatory properties as shown by our in vitro studies. Based on this evidence, we hypothesized that CDDO-Me would reduce lung inflammation, fibrosis and lung function impairment in a bleomycin model of lung injury and fibrosis. To test this hypothesis, mice received bleomycin via oropharyngeal aspiration (OA) on day zero and CDDO-Me during the inflammatory phase from days -1 to 9 every other day. Bronchoalveolar lavage fluid (BALF) and lung tissue were harvested on day 7 to evaluate inflammation, while fibrosis and lung function were evaluated on day 21. On day 7, CDDO-Me reduced total BALF protein by 50{\%}, alveolar macrophage infiltration by 40{\%}, neutrophil infiltration by 90{\%} (p≤0.01), inhibited production of the inflammatory cytokines KC and IL-6 by over 90{\%} (p≤0.001), and excess production of the pro-fibrotic cytokine TGF$\beta$ by 50{\%}. CDDO-Me also inhibited $\alpha$-smooth muscle actin and fibronectin mRNA by 50{\%} (p≤0.05). On day 21, CDDO-Me treatment reduced histological fibrosis, collagen deposition and $\alpha$SMA production. Lung function was significantly improved at day 21 by treatment with CDDO-Me, as demonstrated by respiratory rate and dynamic compliance. These new findings reveal that CDDO-Me exhibits potent anti-fibrotic and anti-inflammatory properties in vivo. CDDO-Me is a potential new class of drugs to arrest inflammation and ameliorate fibrosis in patients who are predisposed to lung injury and fibrosis incited by cancer treatments (e.g. chemotherapy and radiation) and by systemic autoimmune diseases.
Chemical reviews 2005 feb
Antitumor antibiotics: bleomycin, enediynes, and mitomycin.
Abstract
Abstract
Journal of natural products 2000 jan
Bleomycin: new perspectives on the mechanism of action.
Abstract
Abstract
The bleomycin group antitumor antibiotics have long been of interest as a consequence of their efficacy in the treatment of certain tumors, not to mention their unique structures and properties in mediating dioxygen activation and sequence selective degradation of DNA. At a chemical level, the structure originally assigned to bleomycin was subsequently reassigned and the new structure has been confirmed by total synthesis. Through the elaboration of structurally modified bleomycin congeners and fragments, synthetic efforts have also facilitated an understanding of the contribution of individual structural domains in bleomycin to sequence selective DNA binding and cleavage, and have also provided insights into the nature of the chemical processes by which DNA degradation takes place. Within the last several years, it has also become apparent that bleomycin can mediate the oxidative degradation of all major classes of cellular RNAs; it seems entirely plausible that RNA may also represent an important locus of action for this class of antitumor agent. In parallel with ongoing synthetic and mechanistic efforts using classical methods, the study of bleomycins attached to solid supports has been shown to provide important mechanistic insights, and the actual elaboration of modified bleomycins by solid phase synthesis constitutes a logical extension of such efforts.

 网站首页
网站首页