概要
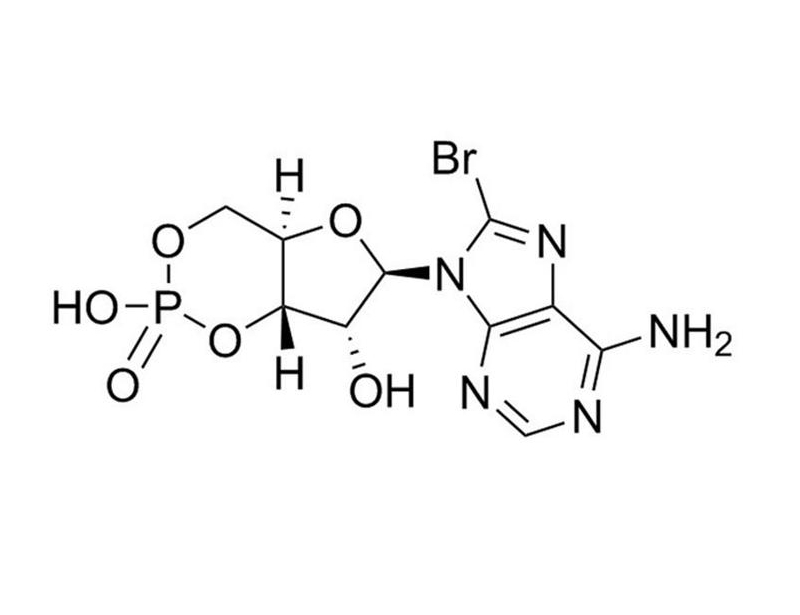
8-Bromo-cAMP is a membrane-permeable cAMP derivative. It can activate cAMP-dependent protein kinase, with long-acting effects due to its resistance to cAMP phosphodiesterase (Schwede et al.). It can be used to study calcium-mediated pathways (IC₅₀ = 0.84 mM; Xaus et al.).
REPROGRAMMING
· Improves the reprogramming efficiency of human neonatal foreskin fibroblast (HFF1) cells, in combination with valproic acid (Wang & Adjaye).
IMMUNOLOGY
· Inhibits M-CSF-dependent proliferation of macrophages (Xaus et al.).
· Protects neutrophils against TNF-α-induced apoptosis (Krakstad).
CANCER RESEARCH
· Induces a proliferative response in an IL-3-dependent leukemic cell line (Barge et al.).
· Induces membrane depolarization in pancreatic cancer cell lines (Sorio et al.).
REPROGRAMMING
· Improves the reprogramming efficiency of human neonatal foreskin fibroblast (HFF1) cells, in combination with valproic acid (Wang & Adjaye).
IMMUNOLOGY
· Inhibits M-CSF-dependent proliferation of macrophages (Xaus et al.).
· Protects neutrophils against TNF-α-induced apoptosis (Krakstad).
CANCER RESEARCH
· Induces a proliferative response in an IL-3-dependent leukemic cell line (Barge et al.).
· Induces membrane depolarization in pancreatic cancer cell lines (Sorio et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | 8-Bromo-cAMP | 73602, 73604 | All | English |
| Safety Data Sheet | 8-Bromo-cAMP | 73602, 73604 | All | English |
数据及文献
Publications (6)
Stem cell reviews 2011 JUN
A cyclic AMP analog, 8-Br-cAMP, enhances the induction of pluripotency in human fibroblast cells.
Abstract
Abstract
Somatic cells can be reprogrammed into induced pluripotent stem (iPS) cells by ectopic expression of four transcription factors. However, the efficiency of human iPS cell generation is extremely low and therefore elucidating the mechanisms underlying cellular reprogramming is of prime importance. We demonstrate that 8-Bromoadenosine 3', 5'-cyclic monophosphate (8-Br-cAMP) improves the reprogramming efficiency of human neonatal foreskin fibroblast (HFF1) cells transduced with the four transcription factors by 2-fold. The combination of 8-Br-cAMP and VPA synergistically increases the efficiency to 6.5-fold. The effect of 8-Br-cAMP or VPA may in part be due to the up-regulation of cytokine-related and inflammatory pathways. Remarkably, the synergistic effect of 8-Br-cAMP and VPA on cellular reprogramming may be due to the transient decrease of p53 protein during the early stages of reprogramming. However, it could also be due to additional differentially regulated genes and pathways such as the up-regulation of cytokine-related, inflammatory pathways and self-renewal supporting gene, namely cyclin-encoding CCND2, and the associated genes CCNA1 and CCNE1. Conversely, we also see the down-regulation of the p53 (CCNB2, GTSE1, SERPINE1) and cell cycle (PLK1, CCNB2) pathways. Our data demonstrates that a cyclic AMP analog, 8-Br-cAMP, enhances the efficiency of cellular reprogramming. In addition, 8-Br-cAMP and VPA have a synergistic effect on cellular reprogramming, which may be in part due to the transient down-regulation of the p53 signaling pathway during the early stages of reprogramming.
PloS one 2011 JAN
Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis.
Abstract
Abstract
BACKGROUND Evaluation of cystic fibrosis transmembrane conductance regulator (CFTR) functional activity to assess new therapies and define diagnosis of cystic fibrosis (CF) is cumbersome. It is known that leukocytes express detectable levels of CFTR but the molecule has not been characterized in these cells. In this study we aim at setting up and validating a blood test to evaluate CFTR expression and function in leukocytes. DESCRIPTION Western blot, PCR, immunofluorescence and cell membrane depolarization analysis by single-cell fluorescence imaging, using the potential-sensitive DiSBAC(2)(3) probe were utilized. Expression of PKA phosphorylated, cell membrane-localized CFTR was detected in non-CF monocytes, being undetectable or present in truncated form in monocytes derived from CF patients presenting with nonsense mutations. CFTR agonist administration induced membrane depolarization in monocytes isolated from non-CF donors (31 subjects) and, to a lesser extent, obligate CFTR heterozygous carriers (HTZ: 15 subjects), but it failed in monocytes from CF patients (44 subjects). We propose an index, which values in CF patients are significantly (ptextless0.001) lower than in the other two groups. Nasal Potential Difference, measured in selected subjects had concordant results with monocytes assay (Kappa statistic 0.93, 95%CI: 0.80-1.00). RESULTS AND SIGNIFICANCE CFTR is detectable and is functional in human monocytes. We also showed that CFTR-associated activity can be evaluated in 5 ml of peripheral blood and devise an index potentially applicable for diagnostic purposes and both basic and translational research: from drug development to evaluation of functional outcomes in clinical trials.
Journal of leukocyte biology 2004 SEP
cAMP protects neutrophils against TNF-alpha-induced apoptosis by activation of cAMP-dependent protein kinase, independently of exchange protein directly activated by cAMP (Epac).
Abstract
Abstract
It is unclear by which receptor cyclic adenosine monophosphate (cAMP) acts to promote neutrophil survival. We found that 8-(4-chlorophenylthio)-2'-O-methyl-cAMP, a specific activator of the recently discovered cAMP receptor, cAMP-regulated guanosine 5'-triphosphate exchange protein directly activated by cAMP, failed to protect human neutrophils from cell death. In contrast, specific activators of cAMP-dependent protein kinase type I (cA-PKI) could protect against death receptor [tumor necrosis factor receptor 1 (TNFR-1), Fas]-mediated apoptosis as well as cycloheximide-accelerated spontaneous" apoptosis. A novel "caged" cA-PK-activating analog�
Pharmacology & therapeutics 2000 JAN
Cyclic nucleotide analogs as biochemical tools and prospective drugs.
Abstract
Abstract
Cyclic AMP (cAMP) and cyclic GMP (cGMP) are key second messengers involved in a multitude of cellular events. From the wealth of synthetic analogs of cAMP and cGMP, only a few have been explored with regard to their therapeutic potential. Some of the first-generation cyclic nucleotide analogs were promising enough to be tested as drugs, for instance N(6),O(2)'-dibutyryl-cAMP and 8-chloro-cAMP (currently in clinical Phase II trials as an anticancer agent). Moreover, 8-bromo and dibutyryl analogs of cAMP and cGMP have become standard tools for investigations of biochemical and physiological signal transduction pathways. The discovery of the Rp-diastereomers of adenosine 3',5'-cyclic monophosphorothioate and guanosine 3',5'-cyclic monophosphorothioate as competitive inhibitors of cAMP- and cGMP-dependent protein kinases, as well as subsequent development of related analogs, has proven very useful for studying the molecular basis of signal transduction. These analogs exhibit a higher membrane permeability, increased resistance against degradation, and improved target specificity. Furthermore, better understanding of signaling pathways and ligand/protein interactions has led to new therapeutic strategies. For instance, Rp-8-bromo-adenosine 3',5'-cyclic monophosphorothioate is employed against diseases of the immune system. This review will focus mainly on recent developments in cyclic nucleotide-related biochemical and pharmacological research, but also highlights some historical findings in the field.
Journal of immunology (Baltimore, Md. : 1950) 1999 OCT
Adenosine inhibits macrophage colony-stimulating factor-dependent proliferation of macrophages through the induction of p27kip-1 expression.
Abstract
Abstract
Adenosine is produced during inflammation and modulates different functional activities in macrophages. In murine bone marrow-derived macrophages, adenosine inhibits M-CSF-dependent proliferation with an IC50 of 45 microM. Only specific agonists that can activate A2B adenosine receptors such as 5'-N-ethylcarboxamidoadenosine, but not those active on A1 (N6-(R)-phenylisopropyladenosine), A2A ([p-(2-carbonylethyl)phenylethylamino]-5'-N-ethylcarboxamido adenosine), or A3 (N6-(3-iodobenzyl)adenosine-5'-N-methyluronamide) receptors, induce the generation of cAMP and modulate macrophage proliferation. This suggests that adenosine regulates macrophage proliferation by interacting with the A2B receptor and subsequently inducing the production of cAMP. In fact, both 8-Br-cAMP (IC50 85 microM) and forskolin (IC50 7 microM) inhibit macrophage proliferation. Moreover, the inhibition of adenylyl cyclase and protein kinase A blocks the inhibitory effect of adenosine and its analogues on macrophage proliferation. Adenosine causes an arrest of macrophages at the G1 phase of the cell cycle without altering the activation of the extracellular-regulated protein kinase pathway. The treatment of macrophages with adenosine induces the expression of p27kip-1, a G1 cyclin-dependent kinase inhibitor, in a protein kinase A-dependent way. Moreover, the involvement of p27kip-1 in the adenosine inhibition of macrophage proliferation was confirmed using macrophages from mice with a disrupted p27kip-1 gene. These results demonstrate that adenosine inhibits macrophage proliferation through a mechanism that involves binding to A2B adenosine receptor, the generation of cAMP, and the induction of p27kip-1 expression.
Biochimica et biophysica acta 1997 FEB
8-Bromo-cAMP induces a proliferative response in an IL-3 dependent leukemic cell line and activates Erk 1,2 via a Shc-independent pathway.
Abstract
Abstract
In a number of cell types, elevation of intracellular cAMP concentrations antagonizes growth factor-induced mitogenesis by abrogating the downstream signaling of RasGTP to extracellular-signal-regulated kinases (Erk 1,2). We studied the effect of elevation of cAMP concentrations on the IL-3-induced mitogenic response in the leukemic cell line AML193. We observed that 8-bromo-cAMP (8-Br-cAMP) had no inhibitory effect on the magnitude of this response. On the contrary. 8-Br-cAMP alone induced a proliferative response in these cells. 8-Br-cAMP activated Erk 1,2 in these cells without involvement of Shc phosphorylation. These findings suggest the presence of a novel cAMP-dependent signaling pathway in AML193 cells, which activates Erk 1,2 via a Shc-independent pathway and leads to the generation of a mitogenic response.

 网站首页
网站首页