概要
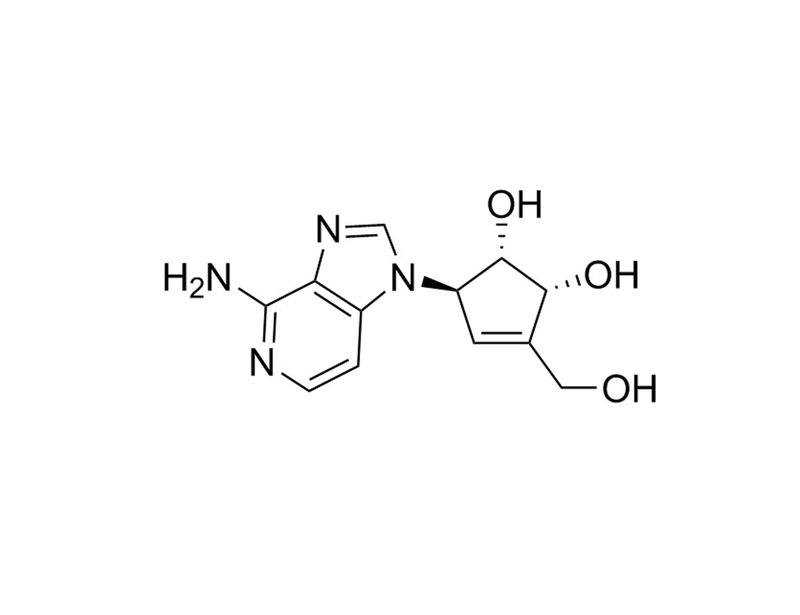
Deazaneplanocin A (DZNep) is an inhibitor of lysine methyltransferases, particularly EZH2. DZNep therefore acts as an epigenetic modifier, specifically inhibiting the trimethylation of Histone 3, Lysine 27, by depleting levels of EZH2. (Miranda et al., Tan et al., Tseng et al.)
REPROGRAMMING
· Enables chemical reprogramming (without genetic factors) of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells, in combination with CHIR99021, Forskolin, Valproic Acid, Tranylcypromine, and E-616452, by increasing OCT4 expression at later stages of reprogramming (Hou et al.).
· Reactivation of XIST-dependent inactive X chromosomes in human embryonic stem cells (Diaz Perez et al.).
CANCER RESEARCH
· Inhibits self-renewal of glioblastoma multiforme cancer stem cells (Suva et al.).
· Inhibits survival of acute myeloid leukemia blast cells, in combination with a histone deacetylase inhibitor (Fiskus et al.).
REPROGRAMMING
· Enables chemical reprogramming (without genetic factors) of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells, in combination with CHIR99021, Forskolin, Valproic Acid, Tranylcypromine, and E-616452, by increasing OCT4 expression at later stages of reprogramming (Hou et al.).
· Reactivation of XIST-dependent inactive X chromosomes in human embryonic stem cells (Diaz Perez et al.).
CANCER RESEARCH
· Inhibits self-renewal of glioblastoma multiforme cancer stem cells (Suva et al.).
· Inhibits survival of acute myeloid leukemia blast cells, in combination with a histone deacetylase inhibitor (Fiskus et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | 3-Deazaneplanocin A | 72322, 72324 | All | English |
| Safety Data Sheet | 3-Deazaneplanocin A | 72322, 72324 | All | English |
数据及文献
Publications (7)
Science (New York, N.Y.) 2013 AUG
Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds.
Abstract
Abstract
Pluripotent stem cells can be induced from somatic cells, providing an unlimited cell resource, with potential for studying disease and use in regenerative medicine. However, genetic manipulation and technically challenging strategies such as nuclear transfer used in reprogramming limit their clinical applications. Here, we show that pluripotent stem cells can be generated from mouse somatic cells at a frequency up to 0.2% using a combination of seven small-molecule compounds. The chemically induced pluripotent stem cells resemble embryonic stem cells in terms of their gene expression profiles, epigenetic status, and potential for differentiation and germline transmission. By using small molecules, exogenous master genes" are dispensable for cell fate reprogramming. This chemical reprogramming strategy has potential use in generating functional desirable cell types for clinical applications."
Human molecular genetics 2012 FEB
Derivation of new human embryonic stem cell lines reveals rapid epigenetic progression in vitro that can be prevented by chemical modification of chromatin.
Abstract
Abstract
Human embryonic stem cells (hESCs) are pluripotent cell types derived from the inner cell mass of human blastocysts. Recent data indicate that the majority of established female XX hESC lines have undergone X chromosome inactivation (XCI) prior to differentiation, and XCI of hESCs can be either XIST-dependent (class II) or XIST-independent (class III). XCI of female hESCs precludes the use of XX hESCs as a cell-based model for examining mechanisms of XCI, and will be a challenge for studying X-linked diseases unless strategies are developed to reactivate the inactive X. In order to recover nuclei with two active X chromosomes (class I), we developed a reprogramming strategy by supplementing hESC media with the small molecules sodium butyrate and 3-deazaneplanocin A (DZNep). Our data demonstrate that successful reprogramming can occur from the XIST-dependent class II nuclear state but not class III nuclear state. To determine whether these small molecules prevent XCI, we derived six new hESC lines under normoxic conditions (UCLA1-UCLA6). We show that class I nuclei are present within the first 20 passages of hESC derivation prior to cryopreservation, and that supplementation with either sodium butyrate or DZNep preserve class I nuclei in the self-renewing state. Together, our data demonstrate that self-renewal and survival of class I nuclei are compatible with normoxic hESC derivation, and that chemical supplementation after derivation provides a strategy to prevent epigenetic progression and retain nuclei with two active X chromosomes in the self-renewing state.
Blood 2009 SEP
Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells.
Abstract
Abstract
The polycomb repressive complex (PRC) 2 contains 3 core proteins, EZH2, SUZ12, and EED, in which the SET (suppressor of variegation-enhancer of zeste-trithorax) domain of EZH2 mediates the histone methyltransferase activity. This induces trimethylation of lysine 27 on histone H3, regulates the expression of HOX genes, and promotes proliferation and aggressiveness of neoplastic cells. In this study, we demonstrate that treatment with the S-adenosylhomocysteine hydrolase inhibitor 3-deazaneplanocin A (DZNep) depletes EZH2 levels, and inhibits trimethylation of lysine 27 on histone H3 in the cultured human acute myeloid leukemia (AML) HL-60 and OCI-AML3 cells and in primary AML cells. DZNep treatment induced p16, p21, p27, and FBXO32 while depleting cyclin E and HOXA9 levels. Similar findings were observed after treatment with small interfering RNA to EZH2. In addition, DZNep treatment induced apoptosis in cultured and primary AML cells. Furthermore, compared with treatment with each agent alone, cotreatment with DZNep and the pan-histone deacetylase inhibitor panobinostat caused more depletion of EZH2, induced more apoptosis of AML, but not normal CD34(+) bone marrow progenitor cells, and significantly improved survival of nonobese diabetic/severe combined immunodeficiency mice with HL-60 leukemia. These findings indicate that the combination of DZNep and panobinostat is effective and relatively selective epigenetic therapy against AML cells.
Molecular cancer therapeutics 2009 JUN
DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation.
Abstract
Abstract
DNA methylation, histone modifications, and nucleosomal occupancy collaborate to cause silencing of tumor-related genes in cancer. The development of drugs that target these processes is therefore important for cancer therapy. Inhibitors of DNA methylation and histone deacetylation have been approved by the Food and Drug Administration for treatment of hematologic malignancies. However, drugs that target other mechanisms still need to be developed. Recently, 3-deazaneplanocin A (DZNep) was reported to selectively inhibit trimethylation of lysine 27 on histone H3 (H3K27me3) and lysine 20 on histone H4 (H4K20me3) as well as reactivate silenced genes in cancer cells. This finding opens the door to the pharmacologic inhibition of histone methylation. We therefore wanted to further study the mechanism of action of DZNep in cancer cells. Western blot analysis shows that DZNep globally inhibits histone methylation and is not selective. Two other drugs, sinefungin and adenosine dialdehyde, have similar effects as DZNep on H3K27me3. Intriguingly, chromatin immunoprecipitation of various histone modifications and microarray analysis show that DZNep acts through a different pathway than 5-aza-2'-deoxycytidine, a DNA methyltransferase inhibitor. These observations give us interesting insight into how chromatin structure affects gene expression. We also determined the kinetics of gene activation to understand if the induced changes were somatically heritable. We found that upon removal of DZNep, gene expression is reduced to its original state. This suggests that there is a homeostatic mechanism that returns the histone modifications to their ground state" after DZNep treatment. Our data show the strong need for further development of histone methylation inhibitors."
Cancer research 2009 DEC
EZH2 is essential for glioblastoma cancer stem cell maintenance.
Abstract
Abstract
Overexpression of the polycomb group protein enhancer of zeste homologue 2 (EZH2) occurs in diverse malignancies, including prostate cancer, breast cancer, and glioblastoma multiforme (GBM). Based on its ability to modulate transcription of key genes implicated in cell cycle control, DNA repair, and cell differentiation, EZH2 is believed to play a crucial role in tissue-specific stem cell maintenance and tumor development. Here, we show that targeted pharmacologic disruption of EZH2 by the S-adenosylhomocysteine hydrolase inhibitor 3-deazaneplanocin A (DZNep), or its specific downregulation by short hairpin RNA (shRNA), strongly impairs GBM cancer stem cell (CSC) self-renewal in vitro and tumor-initiating capacity in vivo. Using genome-wide expression analysis of DZNep-treated GBM CSCs, we found the expression of c-myc, recently reported to be essential for GBM CSCs, to be strongly repressed upon EZH2 depletion. Specific shRNA-mediated downregulation of EZH2 in combination with chromatin immunoprecipitation experiments revealed that c-myc is a direct target of EZH2 in GBM CSCs. Taken together, our observations provide evidence that direct transcriptional regulation of c-myc by EZH2 may constitute a novel mechanism underlying GBM CSC maintenance and suggest that EZH2 may be a valuable new therapeutic target for GBM management.
Genes & development 2007 MAY
Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells.
Abstract
Abstract
Polycomb-repressive complex 2 (PRC2)-mediated histone methylation plays an important role in aberrant cancer gene silencing and is a potential target for cancer therapy. Here we show that S-adenosylhomocysteine hydrolase inhibitor 3-Deazaneplanocin A (DZNep) induces efficient apoptotic cell death in cancer cells but not in normal cells. We found that DZNep effectively depleted cellular levels of PRC2 components EZH2, SUZ12, and EED and inhibited associated histone H3 Lys 27 methylation (but not H3 Lys 9 methylation). By integrating RNA interference (RNAi), genome-wide expression analysis, and chromatin immunoprecipitation (ChIP) studies, we have identified a prominent set of genes selectively repressed by PRC2 in breast cancer that can be reactivated by DZNep. We further demonstrate that the preferential reactivation of a set of these genes by DZNep, including a novel apoptosis affector, FBXO32, contributes to DZNep-induced apoptosis in breast cancer cells. Our results demonstrate the unique feature of DZNep as a novel chromatin remodeling compound and suggest that pharmacologic reversal of PRC2-mediated gene repression by DZNep may constitute a novel approach for cancer therapy.

 网站首页
网站首页