概要
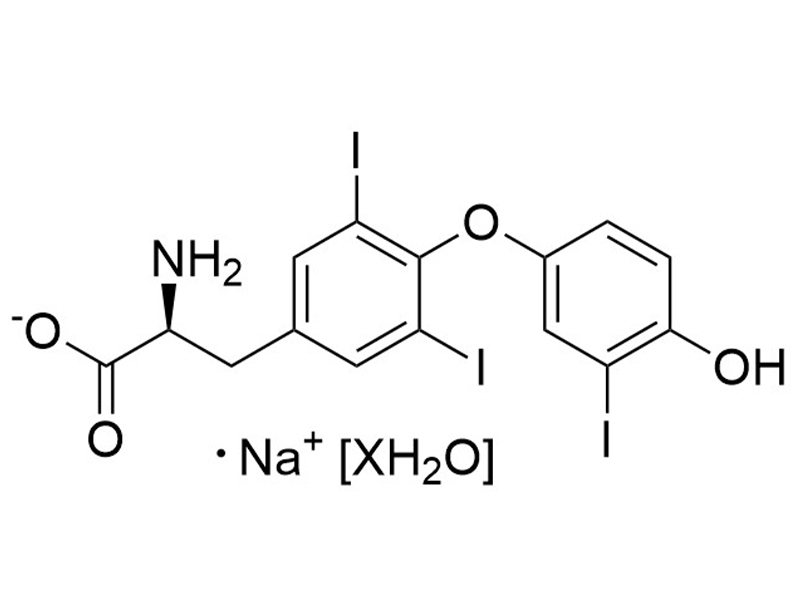
3,3',5-Triiodo-L-thyronine is a thyroid hormone, which is converted from the prohormone thyroxine via deiodination (Misiti et al.). 3,3',5-Triiodo-L-thyronine binds thyroid hormone receptors TRα and TRβ (Kd = 0.06 nM for both thyroid hormone receptors; Sandler et al.), and is essential for growth and differentiation of a variety of cell types (Misiti et al.; Shiohara et al.). 3,3',5-Triiodo-L-thyronine inhibits leucine transport by pituitary cells (IC50 = 2 μM; Yan & Hinkle). This product is supplied as the sodium salt hydrate form of the molecule.
DIFFERENTIATION
· Promotes pancreatic β cell differentiation from human pluripotent stem cells (Pagliuca et al.).
CANCER RESEARCH
· Inhibits the proliferation of pancreatic adenocarcinoma (Michienzi et al.).
DIFFERENTIATION
· Promotes pancreatic β cell differentiation from human pluripotent stem cells (Pagliuca et al.).
CANCER RESEARCH
· Inhibits the proliferation of pancreatic adenocarcinoma (Michienzi et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | 3,3',5-Triiodo-L-thyronine (Sodium Salt Hydrate) | 100-0548, 100-0549 | All | English |
| Safety Data Sheet | 3,3',5-Triiodo-L-thyronine (Sodium Salt Hydrate) | 100-0548, 100-0549 | All | English |
数据及文献
Publications (6)
Cell 2014 oct
Generation of functional human pancreatic $\beta$ cells in vitro.
Abstract
Abstract
The generation of insulin-producing pancreatic $\beta$ cells from stem cells in vitro would provide an unprecedented cell source for drug discovery and cell transplantation therapy in diabetes. However, insulin-producing cells previously generated from human pluripotent stem cells (hPSC) lack many functional characteristics of bona fide $\beta$ cells. Here, we report a scalable differentiation protocol that can generate hundreds of millions of glucose-responsive $\beta$ cells from hPSC in vitro. These stem-cell-derived $\beta$ cells (SC-$\beta$) express markers found in mature $\beta$ cells, flux Ca(2+) in response to glucose, package insulin into secretory granules, and secrete quantities of insulin comparable to adult $\beta$ cells in response to multiple sequential glucose challenges in vitro. Furthermore, these cells secrete human insulin into the serum of mice shortly after transplantation in a glucose-regulated manner, and transplantation of these cells ameliorates hyperglycemia in diabetic mice.
Bioorganic {\&} medicinal chemistry 2012 jun
Discovery of novel indane derivatives as liver-selective thyroid hormone receptor $\beta$ (TR$\beta$) agonists for the treatment of dyslipidemia.
Abstract
Abstract
Thyromimetics that specifically target TR$\beta$ have been shown to reduce plasma cholesterol levels and avoid atherosclerosis through the promotion of reverse cholesterol transport in an animal model. We designed novel thyromimetics with high receptor (TR$\beta$) and organ (liver) selectivity based on the structure of eprotirome (3) and molecular modeling. We found that indane derivatives are potent and dual-selective thyromimetics expected to avoid hypothyroidism in some tissues as well as heart toxicity. KTA-439 (29), a representative indane derivative, showed the same high human TR$\beta$ selectivity in a binding assay as 3 and higher liver selectivity than 3 in a cholesterol-fed rat model.
The Journal of endocrinology 2007 may
3,3',5-Triiodo-L-thyronine inhibits ductal pancreatic adenocarcinoma proliferation improving the cytotoxic effect of chemotherapy.
Abstract
Abstract
The pancreatic adenocarcinoma is an aggressive and devastating disease, which is characterized by invasiveness, rapid progression, and profound resistance to actual treatments, including chemotherapy and radiotherapy. At the moment, surgical resection provides the best possibility for long-term survival, but is feasible only in the minority of patients, when advanced disease chemotherapy is considered, although the effects are modest. Several studies have shown that thyroid hormone, 3,3',5-triiodo-l-thyronine (T(3)) is able to promote or inhibit cell proliferation in a cell type-dependent manner. The aim of the present study is to investigate the ability of T(3) to reduce the cell growth of the human pancreatic duct cell lines chosen, and to increase the effect of chemotherapeutic drugs at conventional concentrations. Three human cell lines hPANC-1, Capan1, and HPAC have been used as experimental models to investigate the T(3) effects on pancreatic adenocarcinoma cell proliferation. The hPANC-1 and Capan1 cell proliferation was significantly reduced, while the hormone treatment was ineffective for HPAC cells. The T(3)-dependent cell growth inhibition was also confirmed by fluorescent activated cell sorting analysis and by cell cycle-related molecule analysis. A synergic effect of T(3) and chemotherapy was demonstrated by cell kinetic experiments performed at different times and by the traditional isobologram method. We have showed that thyroid hormone T(3) and its combination with low doses of gemcitabine (dFdCyd) and cisplatin (DDP) is able to potentiate the cytotoxic action of these chemotherapic drugs. Treatment with 5-fluorouracil was, instead, largely ineffective. In conclusion, our data support the hypothesis that T(3) and its combination with dFdCyd and DDP may act in a synergic way on adenopancreatic ductal cells.
Journal of cellular physiology 2005 jul
3,5,3'-Triiodo-L-thyronine enhances the differentiation of a human pancreatic duct cell line (hPANC-1) towards a beta-cell-Like phenotype.
Abstract
Abstract
The thyroid hormone, 3,5,3'-Triiodo-L-thyronine (T3), is essential for growth, differentiation, and regulation of metabolic functions in multicellular organisms, although the specific mechanisms of this control are still unknown. In this study, treatment of a human pancreatic duct cell line (hPANC-1) with T3 blocks cell growth by an increase of cells in G(0)/G(1) cell cycle phase and enhances morphological and functional changes as indicated by the marked increase in the synthesis of insulin and the parallel decrease of the ductal differentiation marker cytokeratin19. Expression analysis of some of the genes regulating pancreatic beta-cell differentiation revealed a time-dependent increase in insulin and glut2 mRNA levels in response to T3. As last step of the acquisition of a beta-cell-like phenotype, we present evidence that thyroid hormones are able to increase the release of insulin into the culture medium. In conclusion, our results suggest, for the first time, that thyroid hormones induce cell cycle perturbations and play an important role in the process of transdifferentiation of a human pancreatic duct line (hPANC-1) into pancreatic-beta-cell-like cells. These findings have important implications in cell-therapy based treatment of diabetes and may provide important insights in the designing of novel therapeutic agents to restore normal glycemia in subjects with diabetes.
The Journal of biological chemistry 2004 dec
Thyroxine-thyroid hormone receptor interactions.
Abstract
Abstract
Thyroid hormone (TH) actions are mediated by nuclear receptors (TRs alpha and beta) that bind triiodothyronine (T(3), 3,5,3'-triiodo-l-thyronine) with high affinity, and its precursor thyroxine (T(4), 3,5,3',5'-tetraiodo-l-thyronine) with lower affinity. T(4) contains a bulky 5' iodine group absent from T(3). Because T(3) is buried in the core of the ligand binding domain (LBD), we have predicted that TH analogues with 5' substituents should fit poorly into the ligand binding pocket and perhaps behave as antagonists. We therefore examined how T(4) affects TR activity and conformation. We obtained several lines of evidence (ligand dissociation kinetics, migration on hydrophobic interaction columns, and non-denaturing gels) that TR-T(4) complexes adopt a conformation that differs from TR-T(3) complexes in solution. Nonetheless, T(4) behaves as an agonist in vitro (in effects on coregulator and DNA binding) and in cells, when conversion to T(3) does not contribute to agonist activity. We determined x-ray crystal structures of the TRbeta LBD in complex with T(3) and T(4) at 2.5-A and 3.1-A resolution. Comparison of the structures reveals that TRbeta accommodates T(4) through subtle alterations in the loop connecting helices 11 and 12 and amino acid side chains in the pocket, which, together, enlarge a niche that permits helix 12 to pack over the 5' iodine and complete the coactivator binding surface. While T(3) is the major active TH, our results suggest that T(4) could activate nuclear TRs at appropriate concentrations. The ability of TR to adapt to the 5' extension should be considered in TR ligand design.
The Journal of biological chemistry 1993 sep
Saturable, stereospecific transport of 3,5,3'-triiodo-L-thyronine and L-thyroxine into GH4C1 pituitary cells.
Abstract
Abstract
The mechanism of uptake of the thyroid hormones, 3,5,3'-triiodo-L-thyronine (L-T3) and L-thyroxine (L-T4), was studied in rat pituitary GH4C1 cells. The major portion (approximately 65{\%}) of L-T3 transport was stereospecific and saturable. Transport of L-T3 was 8-10 times more rapid than transport of D-T3. [125I]L-T3 transport was saturable at microM concentrations; a Lineweaver-Burk plot was linear with Km = 0.4 microM and Vmax = 4 pmol/min/10(6) cells. Unlabeled analogs competed with [125I]L-T3 uptake in the order L-T3 {\textgreater} or = L-T4 {\textgreater} 3,3',5'-triiodo-L-thyronine (reverse-T3), D-T3, D-T4, and L-thyronine. L-T3 and L-T4 also both effectively inhibited [125I]L-T4 transport. Uptake of [125I]L-T3 was inhibited 40-55{\%} by large neutral amino acids and 77{\%} by 80 microM beta-2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid, an inhibitor selective for the L system of amino acid uptake. Conversely, L-T3 inhibited the transport of [3H]leucine by pituitary cells (IC50 = 2 microM), but D-T3 and 3,5,3'-triiodothyroacetic acid (Triac) did not. L-Leucine was transported much more efficiently (Vmax = 0.65 mumol/min/10(6) cells) than L-T3 by GH4C1 cells. The results show that L-T3 and L-T4 share the same stereospecific transport pathway in pituitary cells, that the transport mechanism is saturable at supraphysiological thyroid hormone concentrations, and that the L system is partially responsible for L-T3 transport.

 网站首页
网站首页