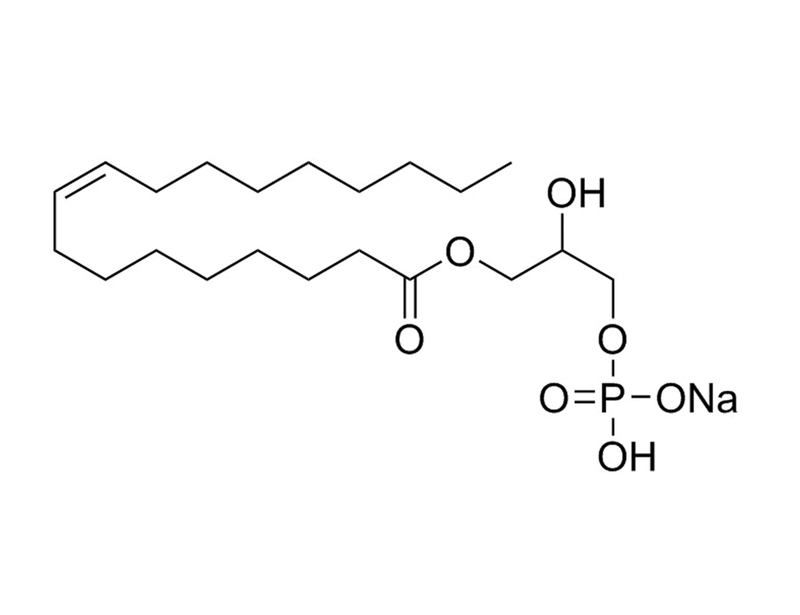
概要
1-Oleoyl Lysophosphatidic Acid is a species of lysophosphatidic acid (LPA) containing oleic acid at the sn-1 position. LPA mediates a variety of biological responses including cell proliferation, smooth muscle contraction, platelet aggregation, neurite retraction, and cell motility (Moolenaar).
DIFFERENTIATION
· Stimulates neuronal differentiation in cultured mouse or rat neural progenitor cells (Cui and Qiao; Fukushima et al.; Spohr et al.).
· Inhibits human embryonic stem (ES) cell-derived neural stem cells (NSCs) from forming neurospheres and differentiating into neurons in vitro (Dottori et al.).
· Stimulates differentiation of human adipose tissue-derived mesenchymal stem cells to myofibroblast-like cells in vitro (Jeon et al.).
DIFFERENTIATION
· Stimulates neuronal differentiation in cultured mouse or rat neural progenitor cells (Cui and Qiao; Fukushima et al.; Spohr et al.).
· Inhibits human embryonic stem (ES) cell-derived neural stem cells (NSCs) from forming neurospheres and differentiating into neurons in vitro (Dottori et al.).
· Stimulates differentiation of human adipose tissue-derived mesenchymal stem cells to myofibroblast-like cells in vitro (Jeon et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | 1-Oleoyl Lysophosphatidic Acid (Sodium Salt) | 72694 | All | English |
数据及文献
Publications (9)
Stem cells (Dayton, Ohio) 2008 MAY
Lysophosphatidic acid inhibits neuronal differentiation of neural stem/progenitor cells derived from human embryonic stem cells.
Abstract
Abstract
Lysophospholipids are signaling molecules that play broad and major roles within the nervous system during both early development and neural injury. We used neural differentiation of human embryonic stem cells (hESC) as an in vitro model to examine the specific effects of lysophosphatidic acid (LPA) at various stages of neural development, from neural induction to mature neurons and glia. We report that LPA inhibits neurosphere formation and the differentiation of neural stem cells (NSC) toward neurons, without modifying NSC proliferation, apoptosis, or astrocytic differentiation. LPA acts through the activation of the Rho/ROCK and the phosphatidylinositol 3-kinase/Akt pathways to inhibit neuronal differentiation. This study is the first demonstration of a role for LPA signaling in neuronal differentiation of hESC. As LPA concentrations increase during inflammation, the inhibition of neuronal differentiation by LPA might contribute to the low level of neurogenesis observed following neurotrauma.
The Journal of biological chemistry 2008 MAR
Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation.
Abstract
Abstract
Lysophosphatidic acid (LPA) is a simple phospholipid derived from cell membranes that has extracellular signaling properties mediated by at least five G protein-coupled receptors referred to as LPA(1)-LPA(5). In the nervous system, receptor-mediated LPA signaling has been demonstrated to influence a range of cellular processes; however, an unaddressed aspect of LPA signaling is its potential to produce specific secondary effects, whereby LPA receptor-expressing cells exposed to, or primed�
Stem cells (Dayton, Ohio) 2008 MAR
Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells.
Abstract
Abstract
Lysophosphatidic acid (LPA) is enriched in ascites of ovarian cancer patients and is involved in growth and invasion of ovarian cancer cells. Accumulating evidence suggests cancer-associated myofibroblasts play a pivotal role in tumorigenesis through secreting stromal cell-derived factor-1 (SDF-1). In the present study, we demonstrate that LPA induces expression of alpha-smooth muscle actin (alpha-SMA), a marker for myofibroblasts, in human adipose tissue-derived mesenchymal stem cells (hADSCs). The LPA-induced expression of alpha-SMA was completely abrogated by pretreatment of the cells with Ki16425, an antagonist of LPA receptors, or by silencing LPA(1) or LPA(2) isoform expression with small interference RNA (siRNA). LPA elicited phosphorylation of Smad2/3, and siRNA-mediated depletion of endogenous Smad2/3 or adenoviral expression of Smad7, an inhibitory Smad, abrogated the LPA induced expression of alpha-SMA and phosphorylation of Smad2/3. LPA-induced secretion of transforming growth factor (TGF)-beta1 in hADSCs, and pretreatment of the cells with SB431542, a TGF-beta type I receptor kinase inhibitor, or anti-TGF-beta1 neutralizing antibody inhibited the LPA-induced expression of alpha-SMA and phosphorylation of Smad2. Furthermore, ascites from ovarian cancer patients or conditioned medium from ovarian cancer cells induced expression of alpha-SMA and phosphorylation of Smad2, and pretreatment of the cells with Ki16425 or SB431542 abrogated the expression of alpha-SMA and phosphorylation of Smad2. In addition, LPA increased the expression of SDF-1 in hADSCs, and pretreatment of the cells with Ki16425 or SB431562 attenuated the LPA-stimulated expression of SDF-1. These results suggest that cancer-derived LPA stimulates differentiation of hADSCs to myofibroblast-like cells and increases SDF-1 expression through activating autocrine TGF-beta1-Smad signaling pathway.
Neurochemistry international 2007 JAN
Lysophosphatidic acid stimulates neuronal differentiation of cortical neuroblasts through the LPA1-G(i/o) pathway.
Abstract
Abstract
Lysophosphatidic acid (LPA) is an extracellular lipid mediator that regulates cortical development. Here we examined how LPA influences the cell fate of cortical neuroblasts using a neurosphere culture system. We generated neurospheres in the presence of basic fibroblast growth factor (bFGF). Treatment with LPA throughout the culture period significantly reduced the number of cells in the neurospheres. When dissociated single cells derived from neurospheres were induced to differentiate by adherence on coverslips, the proportion of MAP2-positive neurons was higher in LPA-treated neurospheres than in those treated with bFGF alone, and the proportion of myelin basic protein-positive oligodendrocytes was lower. Consistent with this finding, LPA raised the ratio of beta-tubulin type III-positive young neurons and reduced the ratio of CD140a-positive oligodendrocyte precursors in neurospheres. These effects of LPA were inhibited by pretreatment of neurospheres with pertussis toxin or an LPA(1)-preferring antagonist, Ki16425. Moreover, LPA-induced enhancement of neuronal differentiation was not observed in neurospheres derived from lpa(1)-null mice. These results suggest that LPA promotes the commitment of neuroblasts to the neural lineage through the LPA(1)-G(i/o) pathway.
Sheng li xue bao : [Acta physiologica Sinica] 2006 DEC
Promotive action of lysophosphatidic acid on proliferation of rat embryonic neural stem cells and their differentiation to cholinergic neurons in vitro.
Abstract
Abstract
Effects of lysophosphatidic acid (LPA), an extracellular phospholipid signal, on the proliferation of rat embryonic neural stem cells (NSCs) and their differentiation into microtubule-associated protein 2 (MAP2)-positive and choline acetyltransferase (ChAT)-positive, i.e. cholinergic-committed neurons, were observed in vitro by [(3)H]-thymidine incorporation, immunocytochemistry, Western blot and other techniques. The results showed that: (1) Lower concentrations of LPA (0.01˜1.0 mumol/L) dose-dependently enhanced the uptake of [(3)H]-thymidine by NSCs cultured in specific serum-free medium, indicating a significant promotive action of LPA on the proliferation of NSCs. (2) After fetal bovine serum which induces and commences the differentiation of NSCs, was used in the medium, the lower concentrations of LPA increased the percentages of both MAP2- and ChAT-immunoreactive neurons, with a peak at 0.1 mumol/L LPA in two cases. (3) The promotive effects of LPA on the differentiation of MAP2- and ChAT-positive neurons were also supported by the up-regulation of the expressions of both MAP2 and ChAT proteins detected by Western blot. (4) At the early phase of differentiation of NSCs, the cell migration and neurite extension were enhanced significantly by lower dosages of LPA under phase-contrast microscope. These results suggest that LPA within certain lower range of concentrations promotes the proliferation of NSCs and their differentiation into unspecific MAP2-positive and specific cholinergic-committed neurons, and also strengthens the migration and neurite extension of the newly-generated neuronal (and also glial as reported elsewhere) progenitors.
Pharmacological reviews 2002 JUN
International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature.
Abstract
Abstract
The lysophospholipids, lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), are now recognized as important extracellular signaling molecules. These lipid mediators are pleiotropic; among the most common cellular responses are mitogenesis, cell survival (anti-apoptosis), inhibition of adenylyl cyclase and calcium mobilization. Physiologic events associated with these mediators include platelet aggregation, vasopressor activity, wound healing, immune modulation, and angiogenesis. Many of the actions of LPA and S1P are mediated through a set of eight G protein-coupled receptors. Five of these are S1P-prefering while the remaining three are LPA receptors. These receptors are expressed widely and in aggregate signal through a variety of heterotrimeric G proteins. The lysophospholipid receptor family is referred to commonly as the Edg" group (e.g.�

 网站首页
网站首页