概要
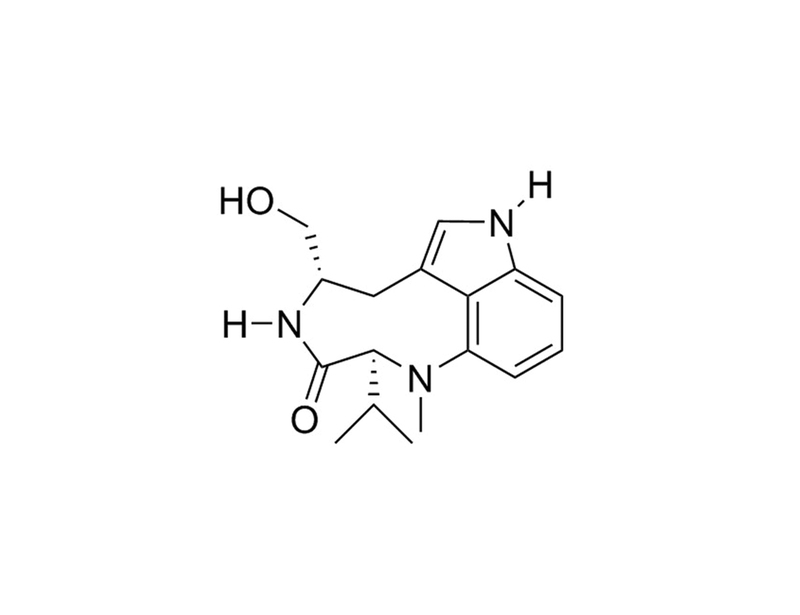
(-)-Indolactam V is an indole alkaloid compound that activates protein kinase C (PKC). It binds to the α, β, γ, δ, ε, and η isozymes of PKC with Ki values of 11, 6, 19, 8, 22, and 16 nM respectively. (Kazanietz et al., Masuda et al.)
DIFFERENTIATION
· Promotes differentiation to human and mouse pancreatic precursors from pluripotent stem cell-derived definitive endoderm (Borowiak et al., Chen et al., Thatava et al.).
DIFFERENTIATION
· Promotes differentiation to human and mouse pancreatic precursors from pluripotent stem cell-derived definitive endoderm (Borowiak et al., Chen et al., Thatava et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | (-)-Indolactam V | 72314 | Catalog #72312: BX29881 or lower; Catalog #72314: BX26035 or lower | English |
| Product Information Sheet 2 | (-)-Indolactam V | 72314 | Catalog #72312: BX33169 or higher; Catalog #72314: BX26036 or higher | English |
| Safety Data Sheet | (-)-Indolactam V | 72314 | All | English |
数据及文献
Publications (5)
Gene therapy 2011 MAR
Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny.
Abstract
Abstract
Nuclear reprogramming of somatic tissue enables derivation of induced pluripotent stem (iPS) cells from an autologous, non-embryonic origin. The purpose of this study was to establish efficient protocols for lineage specification of human iPS cells into functional glucose-responsive, insulin-producing progeny. We generated human iPS cells, which were then guided with recombinant growth factors that mimic the essential signaling for pancreatic development. Reprogrammed with four stemness factors, human fibroblasts were here converted into authentic iPS cells. Under feeder-free conditions, fate specification was initiated with activin A and Wnt3a that triggered engagement into definitive endoderm, followed by priming with fibroblast growth factor 10 (FGF10) and KAAD-cyclopamine. Addition of retinoic acid, boosted by the pancreatic endoderm inducer indolactam V (ILV), yielded pancreatic progenitors expressing pancreatic and duodenal homeobox 1 (PDX1), neurogenin 3 (NGN3) and neurogenic differentiation 1 (NEUROD1) markers. Further guidance, under insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF) and N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), was enhanced by glucagon-like peptide-1 (GLP-1) to generate islet-like cells that expressed pancreas-specific markers including insulin and glucagon. Derived progeny demonstrated sustained expression of PDX1, and functional responsiveness to glucose challenge secreting up to 230 pM of C-peptide. A pancreatogenic cocktail enriched with ILV/GLP-1 offers a proficient means to specify human iPS cells into glucose-responsive hormone-producing progeny, refining the development of a personalized platform for islet-like cell generation.
Nature chemical biology 2009 APR
A small molecule that directs differentiation of human ESCs into the pancreatic lineage.
Abstract
Abstract
Stepwise differentiation from embryonic stem cells (ESCs) to functional insulin-secreting beta cells will identify key steps in beta-cell development and may yet prove useful for transplantation therapy for diabetics. An essential step in this schema is the generation of pancreatic progenitors--cells that express Pdx1 and produce all the cell types of the pancreas. High-content chemical screening identified a small molecule, (-)-indolactam V, that induces differentiation of a substantial number of Pdx1-expressing cells from human ESCs. The Pdx1-expressing cells express other pancreatic markers and contribute to endocrine, exocrine and duct cells, in vitro and in vivo. Further analyses showed that (-)-indolactam V works specifically at one stage of pancreatic development, inducing pancreatic progenitors from definitive endoderm. This study describes a chemical screening platform to investigate human ESC differentiation and demonstrates the generation of a cell population that is a key milepost on the path to making beta cells.
Cell stem cell 2009 APR
Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells.
Abstract
Abstract
An essential step for therapeutic and research applications of stem cells is the ability to differentiate them into specific cell types. Endodermal cell derivatives, including lung, liver, and pancreas, are of interest for regenerative medicine, but efforts to produce these cells have been met with only modest success. In a screen of 4000 compounds, two cell-permeable small molecules were indentified that direct differentiation of ESCs into the endodermal lineage. These compounds induce nearly 80% of ESCs to form definitive endoderm, a higher efficiency than that achieved by Activin A or Nodal, commonly used protein inducers of endoderm. The chemically induced endoderm expresses multiple endodermal markers, can participate in normal development when injected into developing embryos, and can form pancreatic progenitors. The application of small molecules to differentiate mouse and human ESCs into endoderm represents a step toward achieving a reproducible and efficient production of desired ESC derivatives.
Bioscience, biotechnology, and biochemistry 2002 JUL
Binding selectivity of conformationally restricted analogues of (-)-indolactam-V to the C1 domains of protein kinase C isozymes.
Abstract
Abstract
Two conformationally restricted analogues of (-)-indolactam-V (1) (cis and trans amides) were examined for their binding selectivity to the synthetic C1 peptides of all protein kinase C (PKC) isozymes. Although the binding constants of the cis amide-restricted analogue (2) were equal to those of 1, the trans amide-restricted analogue (3) bound significantly only to the novel PKC (delta, epsilon, eta, theta) isozymes.
Molecular pharmacology 1993 AUG
Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes.
Abstract
Abstract
Analysis of [3H]phorbol-12,13-dibutyrate (PDBu) binding was performed with protein kinase C (PKC)-alpha, -beta 1, -gamma, -delta, -epsilon, -eta, and -zeta produced in Sf9 insect cells using the baculovirus expression system. With the exception of PKC-zeta, all of the PKC isozymes bound [3H]PDBu with high affinity (Kd textless 1 nM), either in the presence or in the absence of calcium. Scatchard analysis using 100% phosphatidylserine vesicles revealed slightly lower affinity for the calcium-independent isozymes (PKC-delta, -epsilon, and -eta) than for the calcium-dependent isozymes (PKC-alpha, -beta, and -gamma). Competition for [3H]PDBu binding by different classes of PKC activators showed that 12-deoxyphorbol esters, mezerein, and octahydromezerein likewise possessed lower affinity for the calcium-independent isozymes. The mezerein analog thymeleatoxin was the most marked example, being almost 20-fold less potent for binding to PKC-epsilon and -eta than to PKC-beta 1. In contrast, the indole alkaloids (-)-indolactam V and (-)-octylindolactam V and the postulated endogenous activator 1,2-diacylglycerol bound with similar affinities to all of the PKC isoforms, suggesting that different residues/configurations in the binding sites of the different PKC isozymes might be involved in interaction with the pharmacophore of the activators. The seven PKC isozymes also showed clearly different substrate specificities with exogenous peptide and protein substrates. The heterogeneous behavior of the different members of the PKC family with ligands and substrates may contribute to the heterogeneity of PKC-mediated pathways at the cellular level.

 网站首页
网站首页