概要
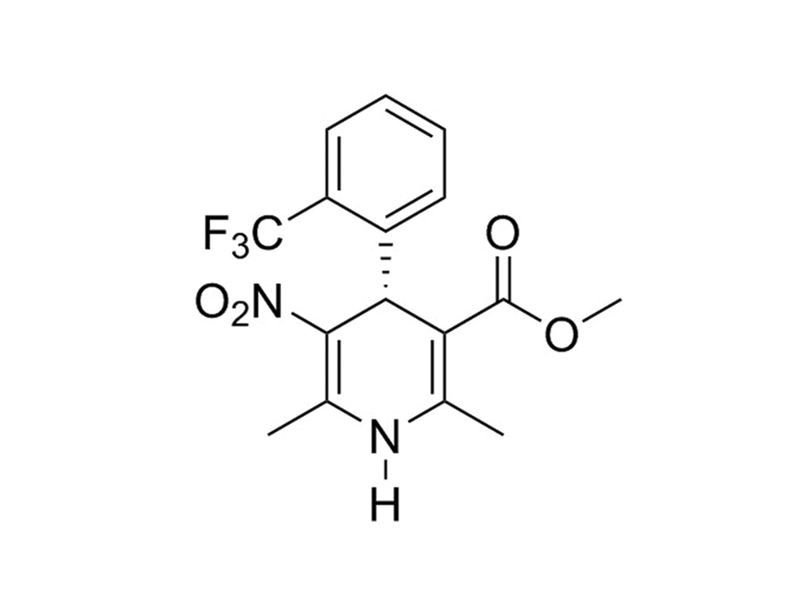
(+)-Bay K8644, a modulator of potential operated calcium channels, exists as two enantiomers that have opposite actions (Hess et al.; Nowycky et al.; O’Neill et al.; Ravens et al.; Yamamoto et al.). (+)-Bay K8644 is an L-type channel blocker that has negative inotropic and vasodilatory effects (Artigas et al.; Franckowiak et al.; Ravens et al.).
REPROGRAMMING
· When combined with BIX-01294, enables reprogramming of mouse embryonic fibroblasts after transduction with OCT4 and KLF4 only (Shi et al.).
DIFFERENTIATION
· Increases neuronal differentiation from neural stem and progenitor cells (NSCs) derived from the brains of postnatal mice (D’Ascenzo et al.).
REPROGRAMMING
· When combined with BIX-01294, enables reprogramming of mouse embryonic fibroblasts after transduction with OCT4 and KLF4 only (Shi et al.).
DIFFERENTIATION
· Increases neuronal differentiation from neural stem and progenitor cells (NSCs) derived from the brains of postnatal mice (D’Ascenzo et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | (+)-Bay K8644 | 72362, 72364 | All | English |
| Safety Data Sheet | (+)-Bay K8644 | 72362, 72364 | All | English |
数据及文献
Publications (9)
Cell stem cell 2008 NOV
Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds.
Abstract
Abstract
Somatic cells can be induced into pluripotent stem cells (iPSCs) with a combination of four transcription factors, Oct4/Sox2/Klf4/c-Myc or Oct4/Sox2/Nanog/LIN28. This provides an enabling platform to obtain patient-specific cells for various therapeutic and research applications. However, several problems remain for this approach to be therapeutically relevant due to drawbacks associated with efficiency and viral genome integration. Recently, it was shown that neural progenitor cells (NPCs) transduced with Oct4/Klf4 can be reprogrammed into iPSCs. However, NPCs express Sox2 endogenously, possibly facilitating reprogramming in the absence of exogenous Sox2. In this study, we identified a small-molecule combination, BIX-01294 and BayK8644, that enables reprogramming of Oct4/Klf4-transduced mouse embryonic fibroblasts, which do not endogenously express the factors essential for reprogramming. This study demonstrates that small molecules identified through a phenotypic screen can compensate for viral transduction of critical factors, such as Sox2, and improve reprogramming efficiency.
The European journal of neuroscience 2006 FEB
Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation.
Abstract
Abstract
Ca(2+) influx through voltage-gated Ca(2+) channels, especially the L-type (Ca(v)1), activates downstream signaling to the nucleus that affects gene expression and, consequently, cell fate. We hypothesized that these Ca(2+) signals may also influence the neuronal differentiation of neural stem/progenitor cells (NSCs) derived from the brain cortex of postnatal mice. We first studied Ca(2+) transients induced by membrane depolarization in Fluo 4-AM-loaded NSCs using confocal microscopy. Undifferentiated cells (nestin(+)) exhibited no detectable Ca(2+) signals whereas, during 12 days of fetal bovine serum-induced differentiation, neurons (beta-III-tubulin(+)/MAP2(+)) displayed time-dependent increases in intracellular Ca(2+) transients, with DeltaF/F ratios ranging from 0.4 on day 3 to 3.3 on day 12. Patch-clamp experiments revealed similar correlation between NSC differentiation and macroscopic Ba(2+) current density. These currents were markedly reduced (-77%) by Ca(v)1 channel blockade with 5 microm nifedipine. To determine the influence of Ca(v)1-mediated Ca(2+) influx on NSC differentiation, cells were cultured in differentiative medium with either nifedipine (5 microm) or the L-channel activator Bay K 8644 (10 microm). The latter treatment significantly increased the percentage of beta-III-tubulin(+)/MAP2(+) cells whereas nifedipine produced opposite effects. Pretreatment with nifedipine also inhibited the functional maturation of neurons, which responded to membrane depolarization with weak Ca(2+) signals. Conversely, Bay K 8644 pretreatment significantly enhanced the percentage of responsive cells and the amplitudes of Ca(2+) transients. These data suggest that NSC differentiation is strongly correlated with the expression of voltage-gated Ca(2+) channels, especially the Ca(v)1, and that Ca(2+) influx through these channels plays a key role in promoting neuronal differentiation.
The Journal of membrane biology 2003 JUN
Effects of the enantiomers of BayK 8644 on the charge movement of L-type Ca channels in guinea-pig ventricular myocytes.
Abstract
Abstract
The effects of the agonist enantiomer S(-)Bay K 8644 on gating charge of L-type Ca channels were studied in single ventricular myocytes. From a holding potential (Vh) of -40 mV, saturating (250 nm) S(-)Bay K shifted the half-distribution voltage of the activation charge (Q1) vs. V curve -7.5 +/- 0.8 mV, almost identical to the shift produced in the Ba conductance vs. V curve (-7.7 +/- 2 mV). The maximum Q1 was reduced by 1.7 +/- 0.2 nC/microF, whereas Q2 (charge moved in inactivated channels) was increased in a similar amount (1.4 +/- 0.4 nC/microF). The steady-state availability curves for Q1, Q2, and Ba current showed almost identical negative shifts of -14.8 +/- 1.7 mV, -18.6 +/- 5.8 mV, and -15.2 +/- 2.7 mV, respectively. The effects of the antagonist enantiomer R(+)BayK 8644 were also studied, the Q1 vs. V curve was not significantly shifted, but Q1max (Vh = -40 mV) was reduced and the Q1 availability curve shifted by -24.6 +/- 1.2 mV. We concluded that: a) the left shift in the Q1 vs. V activation curve produced by S(-)BayK is a purely agonistic effect; b) S(-)BayK induced a significantly larger negative shift in the availability curve than in the Q1 vs. V relation, consistent with a direct promotion of inactivation; c) as expected for a more potent antagonist, R(+)Bay K induced a significantly larger negative shift in the availability curve than did S(-)Bay K.
Naunyn-Schmiedeberg's archives of pharmacology 1990 MAR
Opposite cardiac actions of the enantiomers of Bay K 8644 at different membrane potentials in guinea-pig papillary muscles.
Abstract
Abstract
The influence of membrane potential on the effects of the enantiomers and the racemate of Bay K 8644 [1,4-dihydro-2,6-dimethyl-3-nitro-4-(2-trifluor-methylphenyl)-p yri dine-5-carboxylate] on force of contraction and on action potentials were studied in guinea-pig papillary muscles in order to detect possible changes in the direction of drug action or in potency. Membrane potential was varied by changing the potassium concentration ([K+]o) in the bathing solution. At normal resting potential, (-)-Bay K 8644 enhanced force of contraction and prolonged the action potential duration measured at 50% of repolarization (APD) to the same extent as the racemate and with similar pD2 values. After membrane depolarization by raising [K+]o from 5.4 to 17.4 mmol/l, the (-)-enantiomer and the racemate prolonged the APD to a similar degree but enhanced force to a lesser extent. The maximum rate of depolarization of slow action potentials, Vmax, was increased at the highest concentrations (10(-5) mol/l). The effects of (+)-Bay K 8644 were more complicated. At high concentrations (10(-5) mol/l) it decreased force of contraction and APD, the pD2 values were one order of magnitude lower than for the (-)-enantiomer and the racemate. A high concentration (+)-Bay K 8644 (10(-5) mol/l) virtually abolished contractile activity at all membrane potentials, the extent of shortening in APD increased with membrane depolarization in elevated [K+]o. Vmax of slow action potentials was decreased.(ABSTRACT TRUNCATED AT 250 WORDS)
Brain research bulletin 1988 DEC
Enantiomer selectivity and the development of tolerance to the behavioral effects of the calcium channel activator BAY K 8644.
Abstract
Abstract
The putative behavioral effects of the enantiomers of BAY K 8644 and the behavioral responses to (+/-)-BAY K 8644 following chronic injection were assessed on motor function in mice. The interaction of the enantiomers of BAY K 8644 with mouse brain dihydropyridine binding sites was also evaluated. The calcium channel activating enantiomer (-)-S-BAY K 8644 impaired rotarod and motor activity with an ED50 value of 0.5 mg/kg. The calcium channel blocker enantiomer (+)-R-BAY K 8644 neither affected rotarod nor motor activity. (+)-R-BAY K 8644, and the structurally related dihydropyridine calcium channel blockers nifedipine and (-)-202-791 inhibited the impairment of rotarod activity by (-)-S-BAY K 8644 in a dose-dependent manner. (+/-)-BAY K 8644 produced convulsions in mice with a CD50 of 5 mg/kg. Chronic injection of (+/-)-BAY K 8644 (8 mg/kg IP once each day for four days) resulted in a significant tolerance to, and increase in recovery from, the motor deficits produced by (+/-)-BAY K 8644. Furthermore, chronic treatment with (+/-)-BAY K 8644 increased the onset time, but did not reduce the number of mice having convulsions to (+/-)-BAY K 8644. Chronic injection of nifedipine did not affect the motor deficit and convulsive activity of (+/-)-BAY K 8644. The behavioral effects of (+/-)-BAY K 8644 were observed at significant brain levels of drug. [3H]Nitrendipine binding to mouse brain dihydropyridine binding sites was unchanged in mice chronically injected with either (+/-)-BAY K 8644 or nifedipine.(ABSTRACT TRUNCATED AT 250 WORDS)
European journal of pharmacology 1985 AUG
The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels.
Abstract
Abstract
The optical isomers of the 1,4-dihydropyridine BAY K 8644 were studied in isolated rabbit aorta and heart preparations. The (-)-enantiomer has the known vasoconstricting and positive inotropic properties of the Ca agonistic compound. In contrast, its antipode shows at about 10-50 times higher concentrations the vasodilating and negative inotropic effects of Ca antagonistic drugs. It is concluded that neither simple chemical nor physical actions can be responsible for the opposite effects of Ca antagonistic and Ca agonistic dihydropyridines.

 网站首页
网站首页