概要
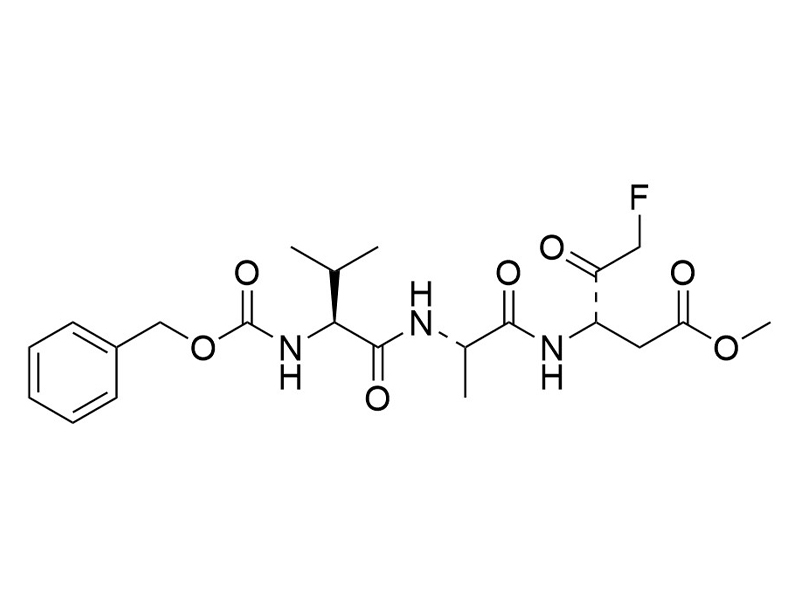
Z-VAD-FMK is a cell-permeable synthetic peptide that inhibits caspase and blocks caspase-mediated apoptosis in vivo (Garcia-Calvo et al.; Xiang et al.). Z-VAD-FMK prevents differentiation and enhances the freeze-thaw survival rate of human embryonic stem cells when subjected to cryopreservation conditions (Heng et al.).
CANCER RESEARCH
· Blocks Fas-induced apoptosis in T lymphocytes (Chow et al.).
CANCER RESEARCH
· Blocks Fas-induced apoptosis in T lymphocytes (Chow et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Z-VAD-FMK | 100-0534, 100-0535 | All | English |
| Safety Data Sheet | Z-VAD-FMK | 100-0534, 100-0535 | All | English |
数据及文献
Publications (4)
Bioscience reports 2007 oct
Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells.
Abstract
Abstract
Previous study demonstrated that the low survival of human embryonic stem cells (hESC) under conventional slow-cooling cryopreservation protocols is predominantly due to apoptosis rather than cellular necrosis. Hence, this study investigated whether a synthetic broad-spectrum irreversible inhibitor of caspase enzymes, Z-VAD-FMK can be used to enhance the post-thaw survival rate of hESC. About 100 mM Z-VAD-FMK was supplemented into either the freezing solution, the post-thaw culture media or both. Intact and adherent hESC colonies were cryopreserved so as to enable subsequent quantitation of the post-thaw cell survival rate through the MTT assay, which can only be performed with adherent cells. Exposure to 100 mM Z-VAD-FMK in the freezing solution alone did not significantly enhance the post-thaw survival rate (10.2{\%} vs. 9.9{\%}, p {\textgreater} 0.05). However, when 100 mM Z-VAD-FMK was added to the post-thaw culture media, there was a significant enhancement in the survival rate from 9.9{\%} to 14.4{\%} (p {\textless} 0.05), which was further increased to 18.7{\%} when Z-VAD-FMK was also added to the freezing solution as well (p {\textless} 0.01). Spontaneous differentiation of hESC after cryopreservation was assessed by morphological observations under bright-field microscopy, and by immunocytochemical staining for the pluripotency markers SSEA-3 and TRA-1-81. The results demonstrated that exposure to Z-VAD-FMK did not significantly enhance the spontaneous differentiation of hESC within post-thaw culture.
The Journal of biological chemistry 1998 dec
Inhibition of human caspases by peptide-based and macromolecular inhibitors.
Abstract
Abstract
Studies with peptide-based and macromolecular inhibitors of the caspase family of cysteine proteases have helped to define a central role for these enzymes in inflammation and mammalian apoptosis. A clear interpretation of these studies has been compromised by an incomplete understanding of the selectivity of these molecules. Here we describe the selectivity of several peptide-based inhibitors and the coxpox serpin CrmA against 10 human caspases. The peptide aldehydes that were examined (Ac-WEHD-CHO, Ac-DEVD-CHO, Ac-YVAD-CHO, t-butoxycarbonyl-IETD-CHO, and t-butoxycarbonyl-AEVD-CHO) included several that contain the optimal tetrapeptide recognition motif for various caspases. These aldehydes display a wide range of selectivities and potencies against these enzymes, with dissociation constants ranging from 75 pM to {\textgreater}10 microM. The halomethyl ketone benzyloxycarbonyl-VAD fluoromethyl ketone is a broad specificity irreversible caspase inhibitor, with second-order inactivation rates that range from 2.9 x 10(2) M-1 s-1 for caspase-2 to 2.8 x 10(5) M-1 s-1 for caspase-1. The results obtained with peptide-based inhibitors are in accord with those predicted from the substrate specificity studies described earlier. The cowpox serpin CrmA is a potent (Ki {\textless} 20 nM) and selective inhibitor of Group I caspases (caspase-1, -4, and -5) and most Group III caspases (caspase-8, -9, and -10), suggesting that this virus facilitates infection through inhibition of both apoptosis and the host inflammatory response.
Proceedings of the National Academy of Sciences of the United States of America 1996 dec
BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases.
Abstract
Abstract
Expression of BAX, without another death stimulus, proved sufficient to induce a common pathway of apoptosis. This included the activation of interleukin 1 beta-converting enzyme (ICE)-like proteases with cleavage of the endogenous substrates poly(ADP ribose) polymerase and D4-GDI (GDP dissociation inhibitor for the rho family), as well as the fluorogenic peptide acetyl-Asp-Glu-Val-Asp-aminotrifluoromethylcoumarin (DEVD-AFC). The inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (zVAD-fmk) successfully blocked this protease activity and prevented FAS-induced death but not BAX-induced death. Blocking ICE-like protease activity prevented the cleavage of nuclear and cytosolic substrates and the DNA degradation that followed BAX induction. However, the fall in mitochondrial membrane potential, production of reactive oxygen species, cytoplasmic vacuolation, and plasma membrane permeability that are downstream of BAX still occurred. Thus, BAX-induced alterations in mitochondrial function and subsequent cell death do not apparently require the known ICE-like proteases.
FEBS letters 1995 may
Involvement of multiple proteases during Fas-mediated apoptosis in T lymphocytes.
Abstract
Abstract
The mechanism of Fas antigen-mediated apoptosis is at present unclear. We show here that the 100,000 x g supernatant from cell lysates prepared from anti-Fas-stimulated JUR-KAT T cells, induces chromatin fragmentation in isolated nuclei with concomitant morphological changes typically seen in apoptosis. The formation of this apoptotic nuclei promoting activity (ANPA) in JURKAT T cells after Fas antigen ligation was blocked by the serine protease inhibitors, TPCK and DCI, and by the interleukin 1-beta-converting enzyme inhibitor, VAD-FMK. In addition, chromatin degradation and morphological changes mediated by the ANPA in isolated nuclei were inhibited by TPCK, but not by DCI or VAD-FMK. These results suggest that Fas-mediated apoptosis in T cells involves the activation of a cascade of proteases.

 网站首页
网站首页