概要
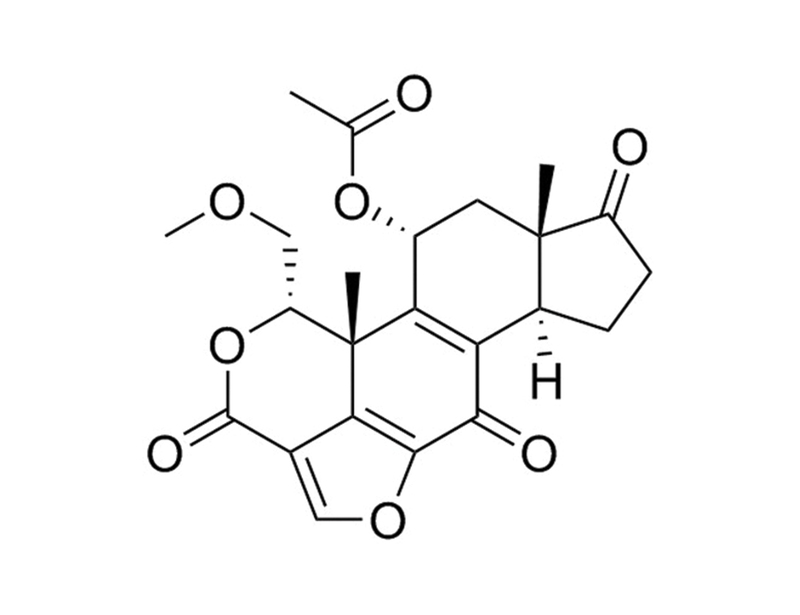
Wortmannin is a fungal metabolite that covalently binds to and inhibits phosphatidylinositol-3-kinases (PI3K) of class I, II, and III. Species-specific differences in the class II PI3Ks determine sensitivity with IC₅₀ = 5, 50, and 450 nM for Drosophila, mouse, and human, respectively (Fruman et al.; Wymann et al.; Okada et al.). Wortmannin also inhibits polo-like kinases (PLK) PLK1 and PLK3 with IC₅₀ = 24 and 49 nM, respectively (Liu et al. 2005; Liu et al. 2007). At high concentrations it can also inhibit other kinases such as mammalian target of rapamycin (mTOR), DNA-dependent protein kinase catalytic subunit (DNA-PKcs), phosphatidylinositol-4-kinase (PI4K), myosin light-chain kinase (MLCK), and mitogen-activated protein kinase (MAPK; Fruman et al.; Meyers & Cantley; Hartley et al.; Brunn et al.; Nakanishi et al.).
CANCER RESEARCH
· Exhibits cytotoxic activity on a number of human tumor cell lines in vitro, and anti-tumor activity in mouse xenografts of C3H mammary carcinoma and BxPC-3 pancreatic carcinoma cells (Schultz et al.; Yuan et al.).
CANCER RESEARCH
· Exhibits cytotoxic activity on a number of human tumor cell lines in vitro, and anti-tumor activity in mouse xenografts of C3H mammary carcinoma and BxPC-3 pancreatic carcinoma cells (Schultz et al.; Yuan et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Wortmannin | 73562, 73564 | All | English |
| Safety Data Sheet | Wortmannin | 73562, 73564 | All | English |
数据及文献
Publications (11)
The Journal of biological chemistry 2007
Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects.
Abstract
Abstract
Polo-like kinases play crucial roles throughout mitosis. We previously reported that wortmannin potently inhibits Polo-like kinase 1 (Plk1). In this study, we show that wortmannin also strongly inhibits Polo-like kinase 3 (Plk3). To further characterize this inhibition, we identified the sites of labeling on Plk1 and Plk3 targeted by AX7503, a tetramethylrhodamine-wortmannin conjugate. AX7503 labeling on Plk1 and Plk3 was found to occur on a conserved ATP binding site residue. In addition, we show that wortmannin inhibits Plk3 activity in live cells at concentrations commonly used to inhibit the more well known targets of wortmannin, the phosphoinositide 3-kinases. Importantly, we found that inhibition of Plk3 by wortmannin lead to a decrease in phosphorylation of p53 on serine 20 induced by DNA damage, demonstrating the effect of wortmannin on a downstream Plk3 target. Taken together, our results suggest that wortmannin can affect multiple functions of Plk3 in cell cycle progression and at the DNA damage check point. The identification of the labeling sites of Plk1 and Plk3 by AX7503 may be useful in designing more effective compounds to target Polo-like kinases for cancer treatment and also may be useful for the structural study of Plk domains.
Chemistry & biology 2007
Covalent reactions of wortmannin under physiological conditions.
Abstract
Abstract
Wortmannin (Wm), a steroid-like molecule of 428.4 Da, appears to be unstable in biological fluids (apparent chemical instability), yet it exhibits an antiproliferative activity in assays employing a 48 hr incubation period (prolonged bioactivity), a situation we refer to as the wortmannin paradox." Under physiological conditions�
Chemistry & biology 2005
Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase.
Abstract
Abstract
Polo-like kinases (PLKs) play critical roles throughout mitosis. Here, we report that wortmannin, which was previously thought to be a highly selective inhibitor of phosphoinositide (PI) 3-kinases, is a potent inhibitor of mammalian PLK1. Observation of the wortmannin-PLK1 interaction was enabled by a tetramethylrhodamine-wortmannin conjugate (AX7503) that permits rapid detection of PLK1 activity and expression in complex proteomes. Importantly, we show that wortmannin inhibits PLK1 activity in an in vitro kinase assay with an IC(50) of 24 nM and when incubated with intact cells. Taken together, our results indicate that, at the concentrations of wortmannin commonly used to inhibit PI 3-kinases, PLK1 is also significantly inhibited.
Annual review of biochemistry 1998
Phosphoinositide kinases.
Abstract
Abstract
Phosphatidylinositol, a component of eukaryotic cell membranes, is unique among phospholipids in that its head group can be phosphorylated at multiple free hydroxyls. Several phosphorylated derivatives of phosphatidylinositol, collectively termed phosphoinositides, have been identified in eukaryotic cells from yeast to mammals. Phosphoinositides are involved in the regulation of diverse cellular processes, including proliferation, survival, cytoskeletal organization, vesicle trafficking, glucose transport, and platelet function. The enzymes that phosphorylate phosphatidylinositol and its derivatives are termed phosphoinositide kinases. Recent advances have challenged previous hypotheses about the substrate selectivity of different phosphoinositide kinase families. Here we re-examine the pathways of phosphoinositide synthesis and the enzymes involved.
The Journal of biological chemistry 1997
Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase.
Abstract
Abstract
Phosphatidylinositol (PtdIns) 4-kinases catalyze the synthesis of PtdIns-4-P, the immediate precursor of PtdIns-4,5-P2. Here we report the cloning of a novel, ubiquitously expressed PtdIns 4-kinase (PI4Kbeta). The 2.4-kilobase pair cDNA encodes a putative translation product of 801 amino acids which shows greatest homology to the yeast PIK1 gene. The recombinant protein exhibits lipid kinase activity when expressed in Escherichia coli, and specific antibodies recognize a 110-kDa PtdIns 4-kinase in cell lysates. The biochemical properties of PI4Kbeta are characteristic of a type III enzyme. Interestingly, both recombinant PI4Kbeta and the endogenous protein are inhibited by 150 nM wortmannin, suggesting that we have cloned the previously described PtdIns 4-kinase that is responsible for regulating the synthesis of agonist-sensitive pools of polyphosphoinositides (Nakanishi, S., Catt, J. K., and Balla, T. (1995) Proc. Natl. Acad. Sci. U. S. A. 92, 5317-5321).
Molecular and cellular biology 1996
Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction.
Abstract
Abstract
Wortmannin at nanomolar concentrations is a potent and specific inhibitor of phosphoinositide (PI) 3-kinase and has been used extensively to demonstrate the role of this enzyme in diverse signal transduction processes. At higher concentrations, wortmannin inhibits the ataxia telangiectasia gene (ATM)-related DNA-dependent protein kinase (DNA-PKcs). We report here the identification of the site of interaction of wortmannin on the catalytic subunit of PI 3-kinase, p110alpha. At physiological pH (6.5 to 8) wortmannin reacted specifically with p110alpha. Phosphatidylinositol-4,5-diphosphate, ATP, and ATP analogs [adenine and 5'-(4-fluorosulfonylbenzoyl)adenine] competed effectively with wortmannin, while substances containing nucleophilic amino acid side chain functions had no effect at the same concentrations. This suggests that the wortmannin target site is localized in proximity to the substrate-binding site and that residues involved in wortmannin binding have an increased nucleophilicity because of their protein environment. Proteolytic fragments of wortmannin-treated, recombinant p110alpha were mapped with anti-wortmannin and anti-p110alpha peptide antibodies, thus limiting the target site within a 10-kDa fragment, colocalizing with the ATP-binding site. Site-directed mutagenesis of all candidate residues within this region showed that only the conservative Lys-802-to-Arg mutation abolished wortmannin binding. Inhibition of PI 3-kinase occurs, therefore, by the formation of an enamine following the attack of Lys-802 on the furan ring (at C-20) of wortmannin. The Lys-802-to-Arg mutant was also unable to bind FSBA and was catalytically inactive in lipid and protein kinase assays, indicating a crucial role for Lys-802 in the phosphotransfer reaction. In contrast, an Arg-916-to-Pro mutation abolished the catalytic activity whereas covalent wortmannin binding remained intact. Our results provide the basis for the design of novel and specific inhibitors of an enzyme family, including PI kinases and ATM-related genes, that play a central role in many physiological processes.

 网站首页
网站首页