概要
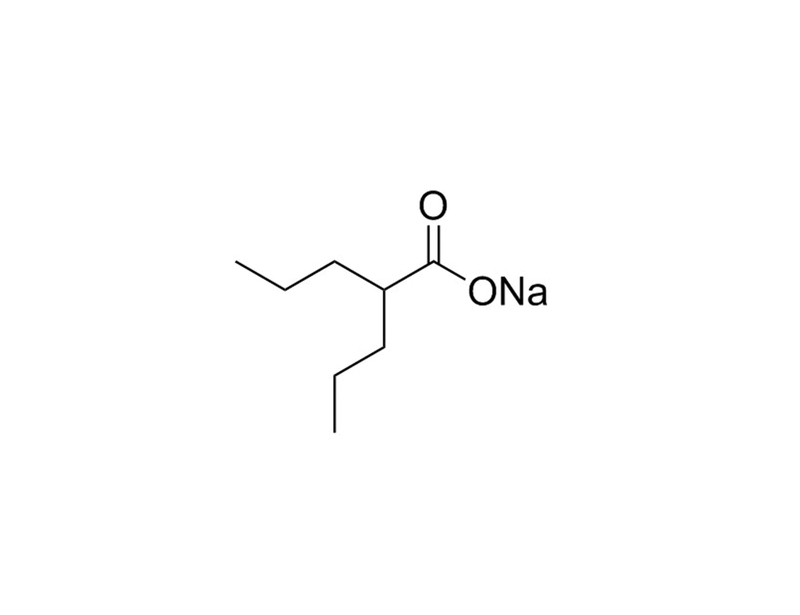
Valproic Acid (VPA) is a short-chain fatty acid that acts as an epigenetic modifier by inhibiting Histone Deacetylases (HDACs) with IC₅₀ values ranging from 0.4 - 3 mM. VPA can also increase γaminobutyric acid (GABA) levels via inhibition of enzymes involved in GABA metabolism. Other effects include depletion of cellular inositol by inhibiting myo-inositol-1-phosphate synthase. (Gottlicher et al., Khan et al., Phiel et al., Rosenberg)
REPROGRAMMING
· Enables chemical reprogramming (without genetic factors) of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells, in combination with CHIR99021, Forskolin, Tranylcypromine, 3-Deazaneplanocin A, and E-616452 (Hou et al.).
· Increases the reprogramming efficiency of mouse embryonic fibroblasts to iPS cells (Huangfu et al. 2008a).
· Promotes reprogramming of human fibroblasts to iPS cells using only 2 factors, OCT4 and SOX2 (Huangfu et al. 2008b).
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, RepSox, Forskolin, SP600125, Gö6983 and Y-27632 (Hu et al.).
MAINTENANCE AND SELF-RENEWAL
· Mediates ex vivo expansion of cord blood CD34+ hematopoietic stem and progenitor cells (Chaurasia et al.).
· Promotes the proliferation and self-renewal of human and mouse hematopoietic progenitor cells (Bug et al., De Felice et al.).
DIFFERENTIATION
· Promotes differentiation of neurons and suppresses differentiation of astrocytes and oligodendrocytes from rat neural progenitor cells (Hsieh et al., Jung et al.).
· Promotes osteogenic differentiation of human mesenchymal stem cells (Cho et al.).
REPROGRAMMING
· Enables chemical reprogramming (without genetic factors) of mouse embryonic fibroblasts to induced pluripotent stem (iPS) cells, in combination with CHIR99021, Forskolin, Tranylcypromine, 3-Deazaneplanocin A, and E-616452 (Hou et al.).
· Increases the reprogramming efficiency of mouse embryonic fibroblasts to iPS cells (Huangfu et al. 2008a).
· Promotes reprogramming of human fibroblasts to iPS cells using only 2 factors, OCT4 and SOX2 (Huangfu et al. 2008b).
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, RepSox, Forskolin, SP600125, Gö6983 and Y-27632 (Hu et al.).
MAINTENANCE AND SELF-RENEWAL
· Mediates ex vivo expansion of cord blood CD34+ hematopoietic stem and progenitor cells (Chaurasia et al.).
· Promotes the proliferation and self-renewal of human and mouse hematopoietic progenitor cells (Bug et al., De Felice et al.).
DIFFERENTIATION
· Promotes differentiation of neurons and suppresses differentiation of astrocytes and oligodendrocytes from rat neural progenitor cells (Hsieh et al., Jung et al.).
· Promotes osteogenic differentiation of human mesenchymal stem cells (Cho et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Valproic Acid (Sodium Salt) | 72292 | All | English |
| Safety Data Sheet | Valproic Acid (Sodium Salt) | 72292 | All | English |
数据及文献
Publications (15)
Leukemia 2016 JAN
Activation of OCT4 enhances ex vivo expansion of human cord blood hematopoietic stem and progenitor cells by regulating HOXB4 expression.
Abstract
Abstract
Although hematopoietic stem cells (HSC) are the best characterized and the most clinically used adult stem cells, efforts are still needed to understand how to best ex vivo expand these cells. Here we present our unexpected finding that OCT4 is involved in the enhancement of cytokine-induced expansion capabilities of human cord blood (CB) HSC. Activation of OCT4 by Oct4-activating compound 1 (OAC1) in CB CD34(+) cells enhanced ex vivo expansion of HSC, as determined by a rigorously defined set of markers for human HSC, and in vivo short-term and long-term repopulating ability in NSG mice. Limiting dilution analysis revealed that OAC1 treatment resulted in 3.5-fold increase in the number of SCID repopulating cells (SRCs) compared with that in day 0 uncultured CD34(+) cells and 6.3-fold increase compared with that in cells treated with control vehicle. Hematopoietic progenitor cells, as assessed by in vitro colony formation, were also enhanced. Furthermore, we showed that OAC1 treatment led to OCT4-mediated upregulation of HOXB4. Consistently, siRNA-mediated knockdown of HOXB4 expression suppressed effects of OAC1 on ex vivo expansion of HSC. Our study has identified the OCT4-HOXB4 axis in ex vivo expansion of human CB HSC.
Cell stem cell 2015 AUG
Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules.
Abstract
Abstract
Neuronal conversion from human fibroblasts can be induced by lineage-specific transcription factors; however, the introduction of ectopic genes limits the therapeutic applications of such induced neurons (iNs). Here, we report that human fibroblasts can be directly converted into neuronal cells by a chemical cocktail of seven small molecules, bypassing a neural progenitor stage. These human chemical-induced neuronal cells (hciNs) resembled hiPSC-derived neurons and human iNs (hiNs) with respect to morphology, gene expression profiles, and electrophysiological properties. This approach was further applied to generate hciNs from familial Alzheimer's disease patients. Taken together, our transgene-free and chemical-only approach for direct reprogramming of human fibroblasts into neurons provides an alternative strategy for modeling neurological diseases and for regenerative medicine.
The Journal of clinical investigation 2014 JUN
Epigenetic reprogramming induces the expansion of cord blood stem cells.
Abstract
Abstract
Cord blood (CB) cells that express CD34 have extensive hematopoietic capacity and rapidly divide ex vivo in the presence of cytokine combinations; however, many of these CB CD34+ cells lose their marrow-repopulating potential. To overcome this decline in function, we treated dividing CB CD34+ cells ex vivo with several histone deacetylase inhibitors (HDACIs). Treatment of CB CD34+ cells with the most active HDACI, valproic acid (VPA), following an initial 16-hour cytokine priming, increased the number of multipotent cells (CD34+CD90+) generated; however, the degree of expansion was substantially greater in the presence of both VPA and cytokines for a full 7 days. Treated CD34+ cells were characterized based on the upregulation of pluripotency genes, increased aldehyde dehydrogenase activity, and enhanced expression of CD90, c-Kit (CD117), integrin α6 (CD49f), and CXCR4 (CD184). Furthermore, siRNA-mediated inhibition of pluripotency gene expression reduced the generation of CD34+CD90+ cells by 89%. Compared with CB CD34+ cells, VPA-treated CD34+ cells produced a greater number of SCID-repopulating cells and established multilineage hematopoiesis in primary and secondary immune-deficient recipient mice. These data indicate that dividing CB CD34+ cells can be epigenetically reprogrammed by treatment with VPA so as to generate greater numbers of functional CB stem cells for use as transplantation grafts.
Science (New York, N.Y.) 2013 AUG
Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds.
Abstract
Abstract
Pluripotent stem cells can be induced from somatic cells, providing an unlimited cell resource, with potential for studying disease and use in regenerative medicine. However, genetic manipulation and technically challenging strategies such as nuclear transfer used in reprogramming limit their clinical applications. Here, we show that pluripotent stem cells can be generated from mouse somatic cells at a frequency up to 0.2% using a combination of seven small-molecule compounds. The chemically induced pluripotent stem cells resemble embryonic stem cells in terms of their gene expression profiles, epigenetic status, and potential for differentiation and germline transmission. By using small molecules, exogenous master genes" are dispensable for cell fate reprogramming. This chemical reprogramming strategy has potential use in generating functional desirable cell types for clinical applications."
BMC cell biology 2008 JAN
Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway.
Abstract
Abstract
BACKGROUND Valproic acid (VPA), a commonly used mood stabilizer that promotes neuronal differentiation, regulates multiple signaling pathways involving extracellular signal-regulated kinase (ERK) and glycogen synthase kinase3beta (GSK3beta). However, the mechanism by which VPA promotes differentiation is not understood. RESULTS We report here that 1 mM VPA simultaneously induces differentiation and reduces proliferation of basic fibroblast growth factor (bFGF)-treated embryonic day 14 (E14) rat cerebral cortex neural progenitor cells (NPCs). The effects of VPA on the regulation of differentiation and inhibition of proliferation occur via the ERK-p21Cip/WAF1 pathway. These effects, however, are not mediated by the pathway involving the epidermal growth factor receptor (EGFR) but via the pathway which stabilizes Ras through beta-catenin signaling. Stimulation of differentiation and inhibition of proliferation in NPCs by VPA occur independently and the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway is involved in both processes. The independent regulation of differentiation and proliferation in NPCs by VPA was also demonstrated in vivo in the cerebral cortex of developing rat embryos. CONCLUSION We propose that this mechanism of VPA action may contribute to an explanation of its anti-tumor and neuroprotective effects, as well as elucidate its role in the independent regulation of differentiation and inhibition of proliferation in the cerebral cortex of developing rat embryos.
The Biochemical journal 2008 JAN
Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors.
Abstract
Abstract
The human HDAC (histone deacetylase) family, a well-validated anticancer target, plays a key role in the control of gene expression through regulation of transcription. While HDACs can be subdivided into three main classes, the class I, class II and class III HDACs (sirtuins), it is presently unclear whether inhibiting multiple HDACs using pan-HDAC inhibitors, or targeting specific isoforms that show aberrant levels in tumours, will prove more effective as an anticancer strategy in the clinic. To address the above issues, we have tested a number of clinically relevant HDACis (HDAC inhibitors) against a panel of rhHDAC (recombinant human HDAC) isoforms. Eight rhHDACs were expressed using a baculoviral system, and a Fluor de Lystrade mark (Biomol International) HDAC assay was optimized for each purified isoform. The potency and selectivity of ten HDACs on class I isoforms (rhHDAC1, rhHDAC2, rhHDAC3 and rhHDAC8) and class II HDAC isoforms (rhHDAC4, rhHDAC6, rhHDAC7 and rhHDAC9) was determined. MS-275 was HDAC1-selective, MGCD0103 was HDAC1- and HDAC2-selective, apicidin was HDAC2- and HDAC3-selective and valproic acid was a specific inhibitor of class I HDACs. The hydroxamic acid-derived compounds (trichostatin A, NVP-LAQ824, panobinostat, ITF2357, vorinostat and belinostat) were potent pan-HDAC inhibitors. The growth-inhibitory effect of the HDACis on HeLa cells showed that both pan-HDAC and class-I-specific inhibitors inhibited cell growth. The results also showed that both pan-HDAC and class-I-specific inhibitor treatment resulted in increased acetylation of histones, but only pan-HDAC inhibitor treatment resulted in increased tubulin acetylation, which is in agreement with their activity towards the HDAC6 isoform.

 网站首页
网站首页