概要
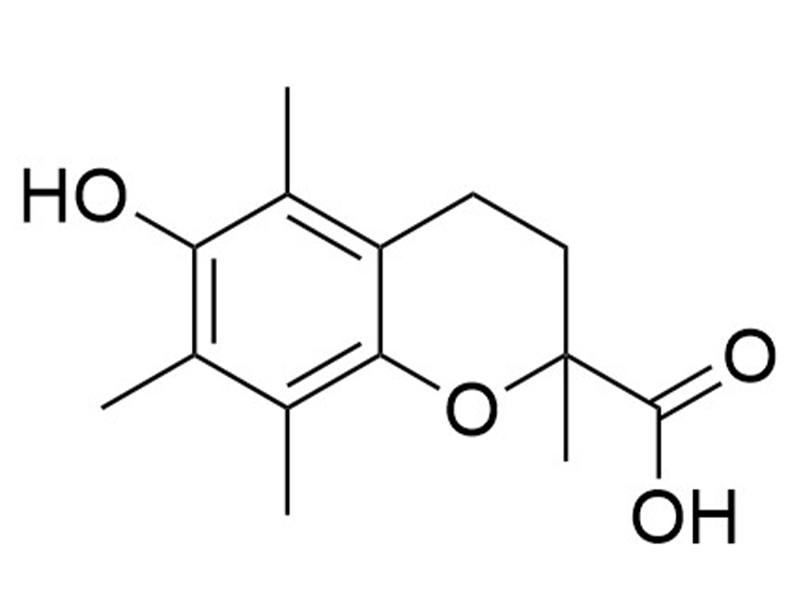
Trolox is a cell-permeable and hydrophilic analog of vitamin E (Choe et al.; Lee et al.). In mice, Trolox has been shown to suppress osteoclast formation by downregulating receptor activator of NF-κB ligand (RANKL) induction and c-Fos expression (Lee et al.). Trolox also prevents cisplatin-induced apoptosis in renal epithelial cells (Xiao et al.).
DIFFERENTIATION
· Induces beta-like cell differentiation from human embryonic stem cells (Petersen et al.).
CANCER RESEARCH
· Enhances arsenic-mediated apoptosis in myeloma and breast cancer cells (Diaz et al.).
DIFFERENTIATION
· Induces beta-like cell differentiation from human embryonic stem cells (Petersen et al.).
CANCER RESEARCH
· Enhances arsenic-mediated apoptosis in myeloma and breast cancer cells (Diaz et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Trolox | 100-0572, 100-0573 | All | English |
| Safety Data Sheet | Trolox | 100-0572, 100-0573 | All | English |
数据及文献
Publications (5)
Cell biology international 2019 jul
Trolox-induced cardiac differentiation is mediated by the inhibition of Wnt/$\beta$-catenin signaling in human embryonic stem cells.
Abstract
Abstract
Cardiac differentiation of human pluripotent stem cells may be induced under chemically defined conditions, wherein the regulation of Wnt/$\beta$-catenin pathway is often desirable. Here, we examined the effect of trolox, a vitamin E analog, on the cardiac differentiation of human embryonic stem cells (hESCs). 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) significantly enhanced cardiac differentiation in a time- and dose-dependent manner after the mesodermal differentiation of hESCs. Trolox promoted hESC cardiac differentiation through its inhibitory activity against the Wnt/$\beta$-catenin pathway. This study demonstrates an efficient cardiac differentiation method and reveals a novel Wnt/$\beta$-catenin regulator.
Stem cell reports 2017
Single-Cell Gene Expression Analysis of a Human ESC Model of Pancreatic Endocrine Development Reveals Different Paths to $\beta$-Cell Differentiation.
Abstract
Abstract
The production of insulin-producing $\beta$ cells from human embryonic stem cells (hESCs) in vitro represents a promising strategy for a cell-based therapy for type 1 diabetes mellitus. To explore the cellular heterogeneity and temporal progression of endocrine progenitors and their progeny, we performed single-cell qPCR on more than 500 cells across several stages of in vitro differentiation of hESCs and compared them with human islets. We reveal distinct subpopulations along the endocrine differentiation path and an early lineage bifurcation toward either polyhormonal cells or $\beta$-like cells. We uncover several similarities and differences with mouse development and reveal that cells can take multiple paths to the same differentiation state, a principle that could be relevant to other systems. Notably, activation of the key $\beta$-cell transcription factor NKX6.1 can be initiated before or after endocrine commitment. The single-cell temporal resolution we provide can be used to improve the production of functional $\beta$ cells.
The Journal of biological chemistry 2009 may
Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling.
Abstract
Abstract
Excessive receptor activator of NF-kappaB ligand (RANKL) signaling causes enhanced osteoclast formation and bone resorption. Thus, down-regulation of RANKL expression or its downstream signals may be a therapeutic approach to the treatment of pathological bone loss. In this study, we investigated the effects of Trolox, a water-soluble vitamin E analogue, on osteoclastogenesis and RANKL signaling. Trolox potently inhibited interleukin-1-induced osteoclast formation in bone marrow cell-osteoblast coculture by abrogating RANKL induction in osteoblasts. This RANKL reduction was attributed to the reduced production of prostaglandin E(2) via a down-regulation of cyclooxygenase-2 activity. We also found that Trolox inhibited osteoclast formation from bone marrow macrophages induced by macrophage colony-stimulating factor plus RANKL in a reversible manner. Trolox was effective only when present during the early stage of culture, which implies that it targets early osteoclast precursors. Pretreatment with Trolox did not affect RANKL-induced early signaling pathways, including MAPKs, NF-kappaB, and Akt. We found that Trolox down-regulated the induction by RANKL of c-Fos protein by suppressing its translation. Ectopic overexpression of c-Fos rescued the inhibition of osteoclastogenesis by Trolox in bone marrow macrophages. Trolox also suppressed interleukin-1-induced osteoclast formation and bone loss in mouse calvarial bone. Taken together, our findings indicate that Trolox prevents osteoclast formation and bone loss by inhibiting both RANKL induction in osteoblasts and c-Fos expression in osteoclast precursors.
Blood 2005 feb
Trolox selectively enhances arsenic-mediated oxidative stress and apoptosis in APL and other malignant cell lines.
Abstract
Abstract
Although arsenic trioxide (As(2)O(3)) is an effective therapy in acute promyelocytic leukemia (APL), its use in other malignancies is limited by the toxicity of concentrations required to induce apoptosis in non-APL tumor cells. We looked for agents that would synergize with As(2)O(3) to induce apoptosis in malignant cells, but not in normal cells. We found that trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a widely known antioxidant, enhances As(2)O(3)-mediated apoptosis in APL, myeloma, and breast cancer cells. Treatment with As(2)O(3) and trolox increased intracellular oxidative stress, as evidenced by heme oxygenase-1 (HO-1) protein levels, c-Jun terminal kinase (JNK) activation, and protein and lipid oxidation. The synergistic effects of trolox may be specific to As(2)O(3), as trolox does not add to toxicity induced by other chemotherapeutic drugs. We explored the mechanism of this synergy using electron paramagnetic resonance and observed the formation of trolox radicals when trolox was combined with As(2)O(3), but not with doxorubicin. Importantly, trolox protected nonmalignant cells from As(2)O(3)-mediated cytotoxicity. Our data provide the first evidence that trolox may extend the therapeutic spectrum of As(2)O(3). Furthermore, the combination of As(2)O(3) and trolox shows potential specificity for tumor cells, suggesting it may not increase the toxicity associated with As(2)O(3) monotherapy in vivo.
Journal of toxicology and environmental health. Part A 2003 mar
Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells.
Abstract
Abstract
Use of cisplatin, a chemotherapeutic agent, is associated with toxicity as a significant number of patients develop a decline in renal function. The mechanisms by which cisplatin produces renal injury are not well understood. It has been suggested that free radical-catalyzed lipid peroxidation can induce apoptosis or necrosis leading to renal injury. This study examined whether low concentrations of cisplatin induce apoptosis in LLC-PK1 cells and whether caspases 1, 2, 3, 8, and 9 are activated during this event. Our results show a dose- and time-dependent induction of apoptosis by micromolar concentrations of cisplatin. Expression of oncogenes c-myc and p53 was induced, and except for caspase 1, all the other caspases tested were activated. Z-VAD, the broad-spectrum inhibitor of caspases, prevented caspase activation and apoptosis, but not c-myc and p53 induction. On the other hand, N-acetylcysteine prevented cisplatin-induced apoptosis as well as c-myc induction but not p53 induction. The antioxidant trolox also prevented cisplatin-induced apoptosis. The results suggest that antioxidants and caspase inhibitors may alleviate cisplatin-associated nephrotoxicity.

 网站首页
网站首页