概要
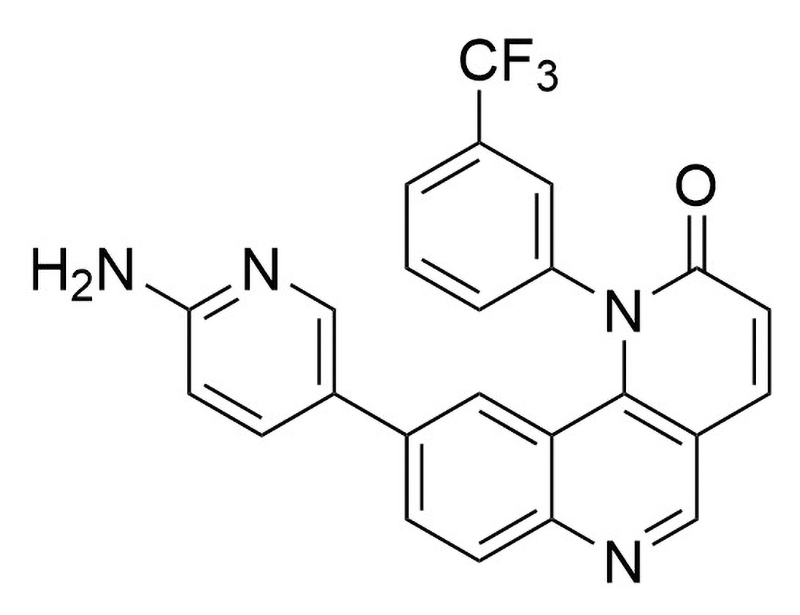
Torin 2 is a potent and selective ATP-competitive inhibitor against mTOR (EC₅₀ = 0.25 nM), a key regulator of cell growth, survival, and autophagy (Liu et al.; Petherick et al.).
CANCER RESEARCH
· Inhibits proliferation of lung, breast, colorectal, and cervical cancer cell lines (Liu et al.).
· Blocks mTOR complex 1 (mTORC1)-associated cell cycle progression and induces autophagy in hepatocellular carcinoma cells (Wang et al.).
CANCER RESEARCH
· Inhibits proliferation of lung, breast, colorectal, and cervical cancer cell lines (Liu et al.).
· Blocks mTOR complex 1 (mTORC1)-associated cell cycle progression and induces autophagy in hepatocellular carcinoma cells (Wang et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Torin 2 | 100-0259, 100-0260 | All | English |
| Safety Data Sheet | Torin 2 | 100-0259, 100-0260 | All | English |
数据及文献
Publications (3)
Oncology reports 2015 oct
The novel mTOR inhibitor Torin-2 induces autophagy and downregulates the expression of UHRF1 to suppress hepatocarcinoma cell growth.
Abstract
Abstract
Mammalian target of rapamycin (mTOR) is frequently upregulated in hepatocellular carcinoma (HCC). Blockage of mTOR was found to induce marked reduction in HCC growth in preclinical models. In the present study, we tested a novel mTOR inhibitor, Torin-2, for its antitumor efficacy in HCC cell lines Hep G2, SNU-182 and Hep 3B2.1-7. The HCC cell lines were cultured in vitro. These cells were treated with Torin-2. Cell apoptosis was evaluated by Annexin V staining. Cell proliferation and cell cycle progression were determined by Ki67 staining and propidium iodide staining, respectively. mTOR signaling, autophagy induction and expression of ubiquitin-like containing PHD and RING finger domains 1 (UHRF1) were assessed by western blot analysis. The UHRF1 mRNA level was determined by real-time PCR. We found that Torin-2 effectively suppressed the growth and survival of HCC cell lines, demonstrated by reduced proliferation and a high rate of apoptosis. Further study elucidated that in addition to blocking mTOR complex 1 (mTORC1)-associated cell cycle progression and induction of autophagy, Torin-2 downregulated transcription of UHRF1, an essential regulator of DNA methylation that is highly expressed in HCC cell lines. Consistently, the level of DNA (cytosine-5)-methyltransferase 1 (DNMT1) was higher after treatment of the HCC cell lines with Torin-2. The downregulation of UHRF1 by Torin-1 was partially due to a decrease in the UHRF1 mRNA level. Torin-2 effectively inhibited HCC cell proliferation through induction of autophagy. Torin‑2-induced downregulation of UHRF1 expression may also contribute to its antitumor effect. Our research provides new clues regarding the antitumor effects of Torin-2 and sheds light on a novel therapeutic approach for HCC.
The Journal of biological chemistry 2015 may
Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy.
Abstract
Abstract
Autophagy is a cell-protective and degradative process that recycles damaged and long-lived cellular components. Cancer cells are thought to take advantage of autophagy to help them to cope with the stress of tumorigenesis; thus targeting autophagy is an attractive therapeutic approach. However, there are currently no specific inhibitors of autophagy. ULK1, a serine/threonine protein kinase, is essential for the initial stages of autophagy, and here we report that two compounds, MRT67307 and MRT68921, potently inhibit ULK1 and ULK2 in vitro and block autophagy in cells. Using a drug-resistant ULK1 mutant, we show that the autophagy-inhibiting capacity of the compounds is specifically through ULK1. ULK1 inhibition results in accumulation of stalled early autophagosomal structures, indicating a role for ULK1 in the maturation of autophagosomes as well as initiation.
Cancer research 2013 apr
Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR.
Abstract
Abstract
mTOR is a highly conserved serine/threonine protein kinase that serves as a central regulator of cell growth, survival, and autophagy. Deregulation of the PI3K/Akt/mTOR signaling pathway occurs commonly in cancer and numerous inhibitors targeting the ATP-binding site of these kinases are currently undergoing clinical evaluation. Here, we report the characterization of Torin2, a second-generation ATP-competitive inhibitor that is potent and selective for mTOR with a superior pharmacokinetic profile to previous inhibitors. Torin2 inhibited mTORC1-dependent T389 phosphorylation on S6K (RPS6KB1) with an EC(50) of 250 pmol/L with approximately 800-fold selectivity for cellular mTOR versus phosphoinositide 3-kinase (PI3K). Torin2 also exhibited potent biochemical and cellular activity against phosphatidylinositol-3 kinase-like kinase (PIKK) family kinases including ATM (EC(50), 28 nmol/L), ATR (EC(50), 35 nmol/L), and DNA-PK (EC(50), 118 nmol/L; PRKDC), the inhibition of which sensitized cells to Irradiation. Similar to the earlier generation compound Torin1 and in contrast to other reported mTOR inhibitors, Torin2 inhibited mTOR kinase and mTORC1 signaling activities in a sustained manner suggestive of a slow dissociation from the kinase. Cancer cell treatment with Torin2 for 24 hours resulted in a prolonged block in negative feedback and consequent T308 phosphorylation on Akt. These effects were associated with strong growth inhibition in vitro. Single-agent treatment with Torin2 in vivo did not yield significant efficacy against KRAS-driven lung tumors, but the combination of Torin2 with mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitor AZD6244 yielded a significant growth inhibition. Taken together, our findings establish Torin2 as a strong candidate for clinical evaluation in a broad number of oncologic settings where mTOR signaling has a pathogenic role.

 网站首页
网站首页