概要
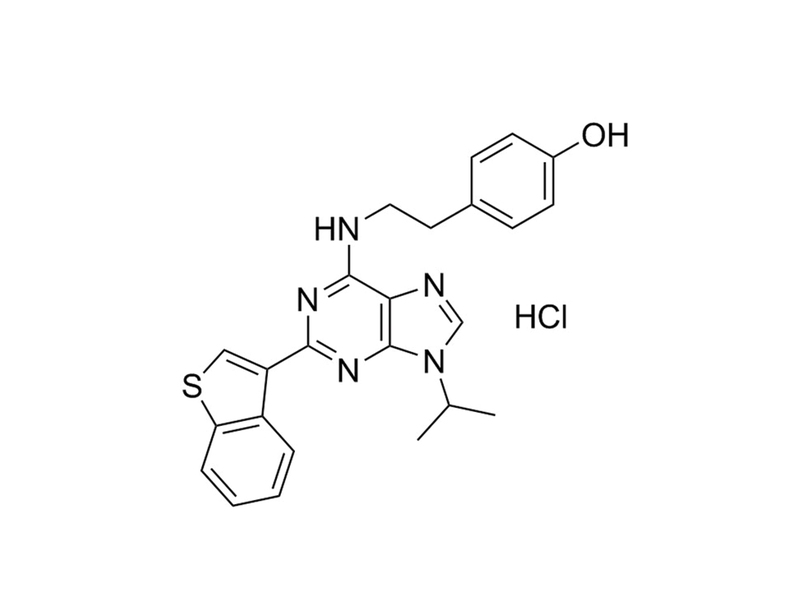
StemRegenin1 (SR1) is an antagonist of the aryl hydrocarbon receptor (AhR). It promotes ex vivo expansion of CD34+ human hematopoietic stem cells and the generation of CD34+ hematopoietic progenitor cells from non-human primate induced pluripotent stem cells. SR1 has been shown to collaborate with UM729 in preventing differentiation of acute myeloid leukemia (AML) cells in culture. SR1 also stimulates the proliferation and differentiation of CD34+ hematopoietic progenitor cells into dendritic cells. This product is supplied as the hydrochloride salt of the molecule.
MAINTENANCE AND SELF-RENEWAL
· Promotes maintenance and expansion of human hematopoietic stem cells in culture (Boitano et al., Csaszar et al.).
DIFFERENTIATION
· Stimulates differentiation of CD34+ hematopoietic progenitor cells into functional human dendritic cells (Thordardottir et al.).
· Promotes hematopoietic differentiation of induced pluripotent stem cells (iPS). (Gori et al.).
CANCER RESEARCH
· Collaborates with UM729 in preventing differentiation of AML cells in culture (Pabst et al.).
MAINTENANCE AND SELF-RENEWAL
· Promotes maintenance and expansion of human hematopoietic stem cells in culture (Boitano et al., Csaszar et al.).
DIFFERENTIATION
· Stimulates differentiation of CD34+ hematopoietic progenitor cells into functional human dendritic cells (Thordardottir et al.).
· Promotes hematopoietic differentiation of induced pluripotent stem cells (iPS). (Gori et al.).
CANCER RESEARCH
· Collaborates with UM729 in preventing differentiation of AML cells in culture (Pabst et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | StemRegenin 1 (Hydrochloride) | 72352, 72354 | All | English |
| Safety Data Sheet | StemRegenin 1 (Hydrochloride) | 72352, 72354 | All | English |
数据及文献
Publications (4)
Stem cells and development 2014 MAY
The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells.
Abstract
Abstract
The superiority of dendritic cells (DCs) as antigen-presenting cells has been exploited in numerous clinical trials, where generally monocyte-derived DCs (Mo-DCs) are injected to induce immunity in patients with cancer or infectious diseases. Despite promising expansion of antigen-specific T cells, the clinical responses following vaccination have been limited, indicating that further improvements of DC vaccine potency are necessary. Pre-clinical studies suggest that vaccination with combination of primary DC subsets, such as myeloid and plasmacytoid blood DCs (mDCs and pDCs, respectively), may result in stronger clinical responses. However, it is a challenge to obtain high enough numbers of primary DCs for immunotherapy, since their frequency in blood is very low. We therefore explored the possibility to generate them from hematopoietic progenitor cells (HPCs). Here, we show that by inhibiting the aryl hydrocarbon receptor with its antagonist StemRegenin 1 (SR1), clinical-scale numbers of functional BDCA2(+)BDCA4(+) pDCs, BDCA1(+) mDCs, and BDCA3(+)DNGR1(+) mDCs can be efficiently generated from human CD34(+) HPCs. The ex vivo-generated DCs were phenotypically and functionally comparable to peripheral blood DCs. They secreted high levels of pro-inflammatory cytokines such as interferon (IFN)-α, interleukin (IL)-12, and tumor necrosis factor (TNF)-α and upregulated co-stimulatory molecules and maturation markers following stimulation with Toll-like receptor (TLR) ligands. Further, they induced potent allogeneic T-cell responses and activated antigen-experienced T cells. These findings demonstrate that SR1 can be exploited to generate high numbers of functional pDCs and mDCs from CD34(+) HPCs, providing an alternative option to Mo-DCs for immunotherapy of patients with cancer or infections.
Blood 2012 SEP
Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells.
Abstract
Abstract
Induced pluripotent stem cell (iPSC) therapeutics are a promising treatment for genetic and infectious diseases. To assess engraftment, risk of neoplastic formation, and therapeutic benefit in an autologous setting, testing iPSC therapeutics in an appropriate model, such as the pigtail macaque (Macaca nemestrina; Mn), is crucial. Here, we developed a chemically defined, scalable, and reproducible specification protocol with bone morphogenetic protein 4, prostaglandin-E2 (PGE2), and StemRegenin 1 (SR1) for hematopoietic differentiation of Mn iPSCs. Sequential coculture with bone morphogenetic protein 4, PGE2, and SR1 led to robust Mn iPSC hematopoietic progenitor cell formation. The combination of PGE2 and SR1 increased CD34(+)CD38(-)Thy1(+)CD45RA(-)CD49f(+) cell yield by 6-fold. CD34(+)CD38(-)Thy1(+)CD45RA(-)CD49f(+) cells isolated on the basis of CD34 expression and cultured in SR1 expanded 3-fold and maintained this long-term repopulating HSC phenotype. Purified CD34(high) cells exhibited 4-fold greater hematopoietic colony-forming potential compared with unsorted hematopoietic progenitors and had bilineage differentiation potential. On the basis of these studies, we calculated the cell yields that must be achieved at each stage to meet a threshold CD34(+) cell dose that is required for engraftment in the pigtail macaque. Our protocol will support scale-up and testing of iPSC-derived CD34(high) cell therapies in a clinically relevant nonhuman primate model.
Cell stem cell 2012 FEB
Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling.
Abstract
Abstract
Clinical hematopoietic transplantation outcomes are strongly correlated with the numbers of cells infused. Anticipated novel therapeutic implementations of hematopoietic stem cells (HSCs) and their derivatives further increase interest in strategies to expand HSCs ex vivo. A fundamental limitation in all HSC-driven culture systems is the rapid generation of differentiating cells and their secreted inhibitory feedback signals. Herein we describe an integrated computational and experimental strategy that enables a tunable reduction in the global levels and impact of paracrine signaling factors in an automated closed-system process by employing a controlled fed-batch media dilution approach. Application of this system to human cord blood cells yielded a rapid (12-day) 11-fold increase of HSCs with self-renewing, multilineage repopulating ability. These results highlight the marked improvements that control of feedback signaling can offer primary stem cell culture and demonstrate a clinically relevant rapid and relatively low culture volume strategy for ex vivo HSC expansion.
Science (New York, N.Y.) 2010 SEP
Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells.
Abstract
Abstract
Although practiced clinically for more than 40 years, the use of hematopoietic stem cell (HSC) transplants remains limited by the ability to expand these cells ex vivo. An unbiased screen with primary human HSCs identified a purine derivative, StemRegenin 1 (SR1), that promotes the ex vivo expansion of CD34+ cells. Culture of HSCs with SR1 led to a 50-fold increase in cells expressing CD34 and a 17-fold increase in cells that retain the ability to engraft immunodeficient mice. Mechanistic studies show that SR1 acts by antagonizing the aryl hydrocarbon receptor (AHR). The identification of SR1 and AHR modulation as a means to induce ex vivo HSC expansion should facilitate the clinical use of HSC therapy.

 网站首页
网站首页