概要
SU11274 is a selective, ATP-competitive inhibitor of MET receptor tyrosine kinase (IC₅₀ = 20 nM; Sattler et al.). It shows good selectivity towards MET versus other receptor tyrosine kinases with IC₅₀ values of 1.3 μM and 9.7 μM for kinase insert domain receptor (KDR) and fibroblast growth factor receptor-1 (FGFR1), respectively (Sattler et al.). It retains activity for certain MET mutants, for example H1112Y and M1268T (Berthou et al.).
CANCER RESEARCH
· Induces apoptosis and cell cycle arrest in TPR-MET-transformed mouse pre-B (Ba/F3) cells (Sattler et al.).
· Inhibits cell viability and motility of human head and neck squamous cell carcinoma cell lines in vitro (Seiwert et al.).
· Inhibits cell viability of c-MET-expressing human non-small cell lung carcinoma cell lines in vitro (Ma et al.).
CANCER RESEARCH
· Induces apoptosis and cell cycle arrest in TPR-MET-transformed mouse pre-B (Ba/F3) cells (Sattler et al.).
· Inhibits cell viability and motility of human head and neck squamous cell carcinoma cell lines in vitro (Seiwert et al.).
· Inhibits cell viability of c-MET-expressing human non-small cell lung carcinoma cell lines in vitro (Ma et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | SU11274 | 73432, 73434 | BX20647 or lower | English |
| Product Information Sheet 2 | SU11274 | 73432, 73434 | BX20648 or higher | English |
| Safety Data Sheet | SU11274 | 73432, 73434 | All | English |
数据及文献
Publications (4)
Cancer research 2009
The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma.
Abstract
Abstract
Recurrent/metastatic head and neck cancer remains a devastating disease with insufficient treatment options. We investigated the MET receptor tyrosine kinase as a novel target for the treatment of head and neck squamous cell carcinoma (HNSCC). MET/phosphorylated MET and HGF expression was analyzed in 121 tissues (HNSCC/normal) by immunohistochemistry, and in 20 HNSCC cell lines by immunoblotting. The effects of MET inhibition using small interfering RNA/two small-molecule inhibitors (SU11274/PF-2341066) on signaling, migration, viability, and angiogenesis were determined. The complete MET gene was sequenced in 66 head and neck cancer tissue samples and eight cell lines. MET gene copy number was determined in 14 cell lines and 23 tumor tissues. Drug combinations of SU11274 with cisplatin or erlotinib were tested in SCC35/HN5 cell lines. Eighty-four percent of the HNSCC samples showed MET overexpression, whereas 18 of 20 HNSCC cell lines (90%) expressed MET. HGF overexpression was present in 45% of HNSCC. MET inhibition with SU11274/PF-2341066 abrogated MET signaling, cell viability, motility/migration in vitro, and tumor angiogenesis in vivo. Mutational analysis of 66 tumor tissues and 8 cell lines identified novel mutations in the semaphorin (T230M/E168D/N375S), juxtamembrane (T1010I/R988C), and tyrosine kinase (T1275I/V1333I) domains (incidence: 13.5%). Increased MET gene copy number was present with textgreater10 copies in 3 of 23 (13%) tumor tissues. A greater-than-additive inhibition of cell growth was observed when combining a MET inhibitor with cisplatin or erlotinib and synergy may be mediated via erbB3/AKT signaling. MET is functionally important in HNSCC with prominent overexpression, increased gene copy number, and mutations. MET inhibition abrogated MET functions, including proliferation, migration/motility, and angiogenesis. MET is a promising, novel target for HNSCC and combination approaches with cisplatin or EGFR inhibitors should be explored.
Cancer research 2005 FEB
Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer.
Abstract
Abstract
Non-small cell lung cancer (NSCLC) is a difficult disease to treat. The c-Met receptor is an attractive potential target for novel therapeutic inhibition in human cancers. We provide strong evidence that c-Met is overexpressed, activated, and sometimes mutated in NSCLC cell lines and tumor tissues. Expression of c-Met was found in all (100%) of the NSCLC tumor tissues examined (n = 23) and most (89%) of the cell lines (n = 9). Sixty-one percent of tumor tissues strongly expressed total c-Met, especially adenocarcinoma (67%). Specific expression of phospho-Met (p-Met) [Y1003] and [Y1230/1234/1235] was seen by immunohistochemistry. p-Met expression was preferentially observed at the NSCLC tumor invasive fronts. c-Met alterations were identified within the semaphorin domain (E168D, L299F, S323G, and N375S) and the juxtamembrane domain (R988C, R988C + T1010I, S1058P, and alternative splice product skipping entire juxtamembrane domain) of a NSCLC cell line and adenocarcinoma tissues. We validated c-Met as potential therapeutic target using small interfering RNA down-regulation of the receptor expression by 50% to 60% in NSCLC cells. This led to inhibition of p-Met and phospho-AKT and up to 57.1 +/- 7.2% cell viability inhibition at 72 hours. The selective small molecule inhibitor of c-Met SU11274 inhibited cell viability in c-Met-expressing NSCLC cells. SU11274 also abrogated hepatocyte growth factor-induced phosphorylation of c-Met and its downstream signaling. Here, we provide first direct evidence by small interfering RNA targeting and small molecule inhibitor that c-Met is important in NSCLC biology and biochemistry. These results indicate that c-Met inhibition will be an important therapeutic strategy against NSCLC to improve its clinical outcome.
Oncogene 2004
The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants.
Abstract
Abstract
Point mutations constitute a major mode of oncogenic activation of the Met receptor tyrosine kinase. Met is aberrantly activated in many types of human malignancies and its deregulated activity is correlated with aggressive tumor traits such as abnormal proliferation and survival, leading to tumor growth, local invasion and metastasis. Here we report that the Met kinase inhibitor SU11274 differentially affects the kinase activity and subsequent signaling of various mutant forms of Met. Two Met variants tested, M1268T and H1112Y, were potently inhibited by 2 microM SU11274, while two other variants, L1213V and Y1248H, remained resistant under similar experimental conditions. Inhibition of the kinase altered cell proliferation, morphology and motility, while cells containing resistant mutants appeared unaffected by the compound. The basis for the sensitivity or resistance to SU11274 is discussed in terms of the position of the mutations predicted from a homology model.
Cancer research 2003
A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase.
Abstract
Abstract
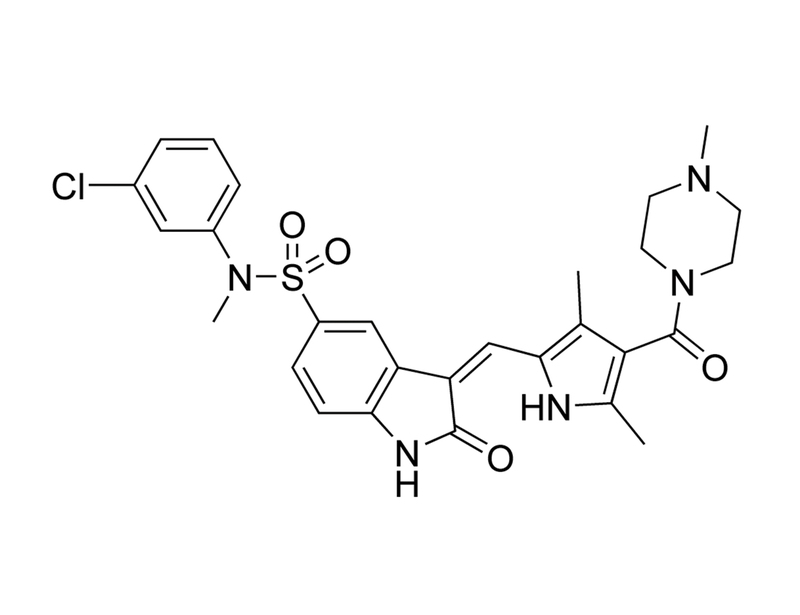
The Met receptor tyrosine kinase has been shown to be overexpressed or mutated in a variety of solid tumors and has, therefore, been identified as a good candidate for molecularly targeted therapy. Activation of the Met tyrosine kinase by the TPR gene was originally described in vitro through carcinogen-induced rearrangement. The TPR-MET fusion protein contains constitutively elevated Met tyrosine kinase activity and constitutes an ideal model to study the transforming activity of the Met kinase. We found, when introduced into an interleukin 3-dependent cell line, TPR-MET induces factor independence and constitutive tyrosine phosphorylation of several cellular proteins. One major tyrosine phosphorylated protein was identified as the TPR-MET oncoprotein itself. Inhibition of the Met kinase activity by the novel small molecule drug SU11274 [(3Z)-N-(3-chlorophenyl)-3-([3,5-dimethyl-4-[(4-methylpiperazin-1-yl)carbonyl]-1H-pyrrol-2-yl]methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide] led to time- and dose-dependent reduced cell growth. The inhibitor did not affect other tyrosine kinase oncoproteins, including BCR-ABL, TEL-JAK2, TEL-PDGFbetaR, or TEL-ABL. The Met inhibitor induced G(1) cell cycle arrest and apoptosis with increased Annexin V staining and caspase 3 activity. The autophosphorylation of the Met kinase was reduced on sites that have been shown previously to be important for activation of pathways involved in cell growth and survival, especially the phosphatidylinositol-3'-kinase and the Ras pathway. In particular, we found that the inhibitor blocked phosphorylation of AKT, GSK-3beta, and the pro-apoptotic transcription factor FKHR. The characterization of SU11274 as an effective inhibitor of Met tyrosine kinase activity illustrates the potential of targeting for Met therapeutic use in cancers associated with activated forms of this kinase.

 网站首页
网站首页