概要
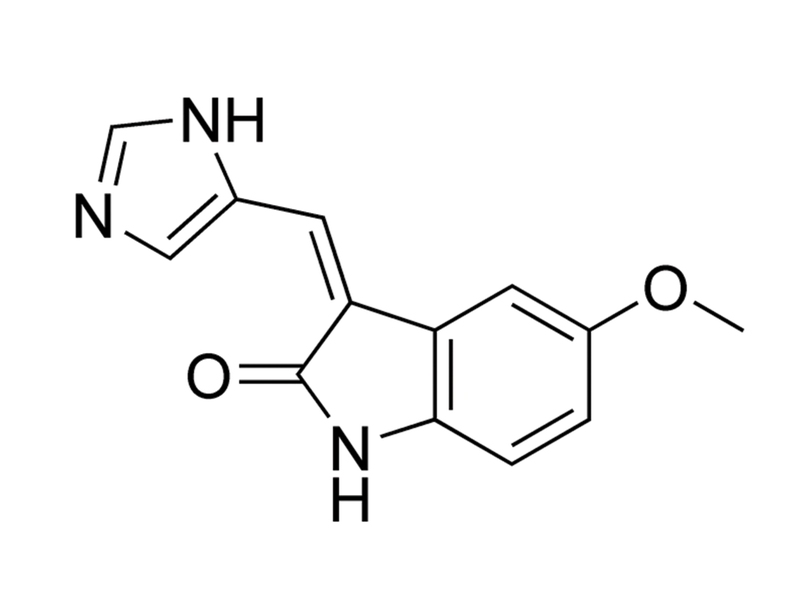
SU9516 is a specific inhibitor of cyclin-dependent kinases (CDKs) including CDK2, CDK1 and, to a lesser extent, CDK4 with IC₅₀ values of 22, 40, and 200 nM, respectively (Lane et al.). It competitively binds in the ATP binding pocket of CDK2 and CDK1 (Moshinsky et al.). It is highly selective and does not inhibit PKC, p38 MAPK, PDGFR or EGFR (IC₅₀ > 10 µM; Lane et al.).
CANCER RESEARCH
· Decreases proliferation in human colon carcinoma cell lines RKO and SW480, through inhibition of retinoblastoma protein (Rb) phosphorylation, resulting in increased Rb/E2F, cell-cycle arrest, and subsequent apoptosis (Lane et al.; Yu et al.).
· Induces mitochondrial injury, caspase activation and subsequent apoptosis through downregulated transcription of the antiapoptotic protein MCL-1 in U937, Jurkat, and HL-60 leukemia or lymphoma cell lines (Gao et al.).
· Induces apoptosis, alone or in combination with paclitaxel, in the inflammatory breast cancer cell line SUM149PT (Opyrchal et al.).
CANCER RESEARCH
· Decreases proliferation in human colon carcinoma cell lines RKO and SW480, through inhibition of retinoblastoma protein (Rb) phosphorylation, resulting in increased Rb/E2F, cell-cycle arrest, and subsequent apoptosis (Lane et al.; Yu et al.).
· Induces mitochondrial injury, caspase activation and subsequent apoptosis through downregulated transcription of the antiapoptotic protein MCL-1 in U937, Jurkat, and HL-60 leukemia or lymphoma cell lines (Gao et al.).
· Induces apoptosis, alone or in combination with paclitaxel, in the inflammatory breast cancer cell line SUM149PT (Opyrchal et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | SU9516 | 73452 | All | English |
| Safety Data Sheet | SU9516 | 73452 | All | English |
数据及文献
Publications (5)
International journal of oncology 2014
Inhibition of Cdk2 kinase activity selectively targets the CD44�?�/CD24�?�/Low stem-like subpopulation and restores chemosensitivity of SUM149PT triple-negative breast cancer cells.
Abstract
Abstract
Inflammatory breast cancer (IBC) is an angioinvasive and most aggressive type of advanced breast cancer characterized by rapid proliferation, chemoresistance, early metastatic development and poor prognosis. IBC tumors display a triple-negative breast cancer (TNBC) phenotype characterized by centrosome amplification, high grade of chromosomal instability (CIN) and low levels of expression of estrogen receptor α (ERα), progesterone receptor (PR) and HER-2 tyrosine kinase receptor. Since the TNBC cells lack these receptors necessary to promote tumor growth, common treatments such as endocrine therapy and molecular targeting of HER-2 receptor are ineffective for this subtype of breast cancer. To date, not a single targeted therapy has been approved for non-inflammatory and inflammatory TNBC tumors and combination of conventional cytotoxic chemotherapeutic agents remains the standard therapy. IBC tumors generally display activation of epithelial to mesenchymal transition (EMT) that is functionally linked to a CD44+/CD24-/Low stem-like phenotype. Development of EMT and consequent activation of stemness programming is responsible for invasion, tumor self-renewal and drug resistance leading to breast cancer progression, distant metastases and poor prognosis. In this study, we employed the luminal ER+ MCF-7 and the IBC SUM149PT breast cancer cell lines to establish the extent to which high grade of CIN and chemoresistance were mechanistically linked to the enrichment of CD44+/CD24low/- CSCs. Here, we demonstrate that SUM149PT cells displayed higher CIN than MCF-7 cells characterized by higher percentage of structural and numerical chromosomal aberrations. Moreover, centrosome amplification, cyclin E overexpression and phosphorylation of retinoblastoma (Rb) were restricted to the stem-like CD44+/CD24-/Low subpopulation isolated from SUM149PT cells. Significantly, CD44+/CD24-/Low CSCs displayed resistance to conventional chemotherapy but higher sensitivity to SU9516, a specific cyclin-dependent kinase 2 (Cdk2) inhibitor, demonstrating that aberrant activation of cyclin E/Cdk2 oncogenic signaling is essential for the maintenance and expansion of CD44+/CD24-/Low CSC subpopulation in IBC. In conclusion, our findings propose a novel therapeutic approach to restore chemosensitivity and delay recurrence of IBC tumors based on the combination of conventional chemotherapy with small molecule inhibitors of the Cdk2 cell cycle kinase.
Molecular pharmacology 2006
The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism.
Abstract
Abstract
Mechanisms of lethality of the three-substituted indolinone and putatively selective cyclin-dependent kinase (CDK)2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516) were examined in human leukemia cells. Exposure of U937 and other leukemia cells to SU9516 concentrations textgreater or =5 microM rapidly (i.e., within 4 h) induced cytochrome c release, Bax mitochondrial translocation, and apoptosis in association with pronounced down-regulation of the antiapoptotic protein Mcl-1. These effects were associated with inhibition of phosphorylation of the carboxyl-terminal domain (CTD) of RNA polymerase (Pol) II on serine 2 but not serine 5. Reverse transcription-polymerase chain reaction analysis revealed pronounced down-regulation of Mcl-1 mRNA levels in SU9516-treated cells. Similar results were obtained in Jurkat and HL-60 leukemia cells. Furthermore, cotreatment with the proteasome inhibitor N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal (MG132) blocked SU9516-mediated Mcl-1 down-regulation, implicating proteasomal degradation in diminished expression of this protein. Ectopic expression of Mcl-1 largely blocked SU9516-induced cytochrome c release, Bax translocation, and apoptosis, whereas knockdown of Mcl-1 by small interfering RNA potentiated SU9516 lethality, confirming the functional contribution of Mcl-1 down-regulation to SU9516-induced cell death. It is noteworthy that SU9516 treatment resulted in a marked increase in reactive oxygen species production, which was diminished, along with cell death, by the free radical scavenger N-acetylcysteine (NAC). We were surprised to find that NAC blocked SU9516-mediated inhibition of RNA Pol II CTD phosphorylation on serine 2, reductions in Mcl-1 mRNA levels, and Mcl-1 down-regulation. Together, these findings suggest that SU9516 kills leukemic cells through inhibition of RNA Pol II CTD phosphorylation in association with oxidative damage and down-regulation of Mcl-1 at the transcriptional level, culminating in mitochondrial injury and cell death.
Biochemical and biophysical research communications 2003
SU9516: biochemical analysis of cdk inhibition and crystal structure in complex with cdk2.
Abstract
Abstract
SU9516 is a 3-substituted indolinone compound with demonstrated potent and selective inhibition toward cyclin dependent kinases (cdks). Here, we describe the kinetic characterization of this inhibition with respect to cdk2, 1, and 4, along with the crystal structure in complex with cdk2. The molecule is competitive with respect to ATP for cdk2/cyclin A, with a K(i) value of 0.031 microM. Similarly, SU9516 inhibits cdk2/cyclin E and cdk1/cyclin B1 in an ATP-competitive manner, although at a 2- to 8-fold reduced potency. In contrast, the compound exhibited non-competitive inhibition with respect to ATP toward cdk4/cyclin D1, with a 45-fold reduced potency. The X-ray crystal structure of SU9516 bound to cdk2 revealed interactions between the molecule and Leu83 and Glu81 of the kinase. This study should aid in the development of more potent and selective cdk inhibitors for potential therapeutic agents.
Biochemical pharmacology 2002
SU9516, a cyclin-dependent kinase 2 inhibitor, promotes accumulation of high molecular weight E2F complexes in human colon carcinoma cells.
Abstract
Abstract
The E2F family plays a critical role in the expression of genes required for entry into and progression through S phase. E2F-mediated transcription is repressed by the tumor suppressor retinoblastoma protein (pRb), which results in sequestration of E2F in a multiprotein complex that includes pRb. Derepression of E2F results from a series of complex phosphorylation events mediated by cyclin D/cdk4 and cyclin E/cdk2. We have employed a novel 3-substituted indolinone compound, 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516), which selectively inhibits cdk2 activity (Lane et al., Cancer Res 2001;61:6170-7) to investigate these events. Electrophoretic mobility gel shift assays were performed on SU9516-treated and -untreated HT-29, SW480, and RKO human colon cancer cell extracts. Treatment with 5 microM SU9516 prevented dissociation of pRb from E2F1 in all cell lines (HT-29textgreaterRKOtextgreaterSW480). Treatment effects were time-dependent, demonstrating greater inhibition at 48 hr versus 24hr in HT-29 cells. Furthermore, E2F species were sequestered in complexes with p107, p130, DP-1, and cyclins A and E. After a 24-hr treatment with 5 microM SU9516, cyclin D1 and cdk2 levels decreased by 10-60%. These findings delineate a previously undescribed mechanism for SU9516-mediated cell growth arrest through down-regulation of cyclin D1, inhibition of cdk2 levels and activity, and pan-sequestration of E2F.
Cancer research 2001
A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells.
Abstract
Abstract
Recent studies have indicated that the development of cyclin-dependent kinase (cdk)2 inhibitors that deregulate E2F are a plausible pharmacological strategy for novel antineoplastic agents. We show here that 3-[1-(3H-Imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516), a novel 3-substituted indolinone compound, binds to and selectively inhibits the activity of cdk2. This inhibition results in a time-dependent decrease (4-64%) in the phosphorylation of the retinoblastoma protein pRb, an increase in caspase-3 activation (5-84%), and alterations in cell cycle resulting in either a G(0)-G(1) or a G(2)-M block. We also report here cell line differences in the cdk-dependent phosphorylation of pRb. These findings demonstrate that SU9516 is a selective cdk2 inhibitor and support the theory that compounds that inhibit cdk2 are viable resources in the development of new antineoplastic agents.

 网站首页
网站首页