概要
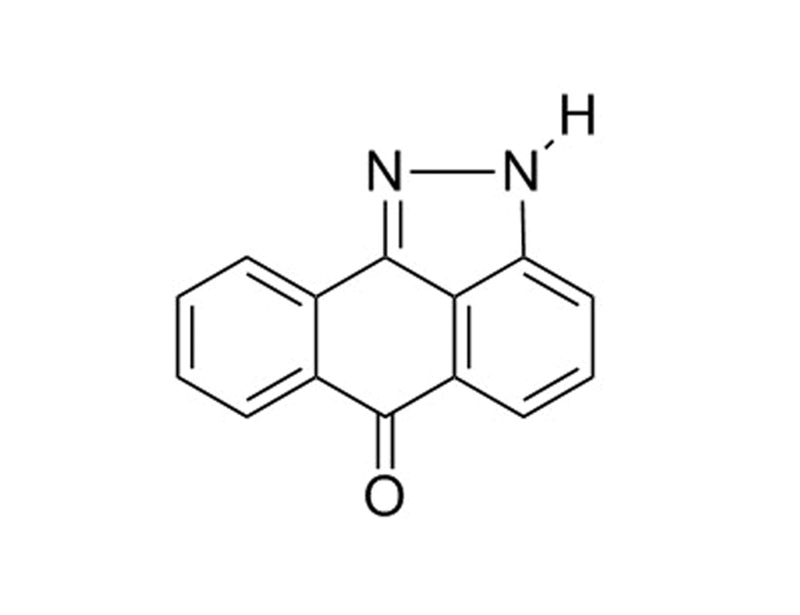
SP600125 is a potent and reversible inhibitor of JNK1-3 (IC₅₀ = 0.11 μM) (Bennett et al.). It is cell-permeable and dose-dependently inhibits c-JUN phosphorylation in cells, blocking the expression of COX-2 and TNF-α in monocytes and IL-10, TNF-α, and IFN- in T-cells (Bennett et al.).
REPROGRAMMING
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, RepSox, Forskolin, Gö6983, Valproic Acid and Y-27632 (Hu et al.).
DIFFERENTIATION
· Inhibits BMP9-induced osteogenic differentiation in cultured mouse mesenchymal stem cells (MSCs) and in primary bone marrow stromal cells (Zhao et al.).
· Promotes adipogenic, but represses osteogenic differentiation of human MSCs (Bilkovski et al.; Liu et al.; Qiu et al.; Tominaga et al.).
· Causes cell death and inhibits neurogenesis when added during early stages of neuronal culture (Tiwari et al.).
REPROGRAMMING
· Direct lineage reprogramming of fibroblasts to mature neurons, in combination with CHIR99021, RepSox, Forskolin, Gö6983, Valproic Acid and Y-27632 (Hu et al.).
DIFFERENTIATION
· Inhibits BMP9-induced osteogenic differentiation in cultured mouse mesenchymal stem cells (MSCs) and in primary bone marrow stromal cells (Zhao et al.).
· Promotes adipogenic, but represses osteogenic differentiation of human MSCs (Bilkovski et al.; Liu et al.; Qiu et al.; Tominaga et al.).
· Causes cell death and inhibits neurogenesis when added during early stages of neuronal culture (Tiwari et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | SP600125 | 72642 | All | English |
| Safety Data Sheet | SP600125 | 72642 | All | English |
数据及文献
Publications (8)
Cell stem cell 2015 AUG
Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules.
Abstract
Abstract
Neuronal conversion from human fibroblasts can be induced by lineage-specific transcription factors; however, the introduction of ectopic genes limits the therapeutic applications of such induced neurons (iNs). Here, we report that human fibroblasts can be directly converted into neuronal cells by a chemical cocktail of seven small molecules, bypassing a neural progenitor stage. These human chemical-induced neuronal cells (hciNs) resembled hiPSC-derived neurons and human iNs (hiNs) with respect to morphology, gene expression profiles, and electrophysiological properties. This approach was further applied to generate hciNs from familial Alzheimer's disease patients. Taken together, our transgene-free and chemical-only approach for direct reprogramming of human fibroblasts into neurons provides an alternative strategy for modeling neurological diseases and for regenerative medicine.
BMB reports 2013 AUG
Activation of JNKs is essential for BMP9-induced osteogenic differentiation of mesenchymal stem cells.
Abstract
Abstract
Although BMP9 is highly capable of promoting osteogenic differentiation of mesenchymal stem cell (MSCs), the molecular mechanism involved remains to be fully elucidated. Here, we explore the possible involvement and detail role of JNKs (c-Jun N-terminal kinases) in BMP9-induced osteogenic differentiation of MSCs. It was found that BMP9 stimulated the activation of JNKs in MSCs. BMP9-induced osteogenic differentiation of MSCs was dramatically inhibited by JNKs inhibitor SP600125. Moreover, BMP9-activated Smads signaling was decreased by SP600125 treatment in MSCs. The effects of inhibitor are reproduced with adenoviruses expressing siRNA targeted JNKs. Taken together, our results revealed that JNKs was activated in BMP9-induced osteogenic differentiation of MSCs. What is most noteworthy, however, is that inhibition of JNKs activity resulted in reduction of BMP9-induced osteogenic differentiation of MSCs, implying that activation of JNKs is essential for BMP9 osteoinductive activity.
Nature genetics 2012 JAN
A chromatin-modifying function of JNK during stem cell differentiation.
Abstract
Abstract
Signaling mediates cellular responses to extracellular stimuli. The c-Jun NH(2)-terminal kinase (JNK) pathway exemplifies one subgroup of the mitogen-activated protein (MAP) kinases, which, besides having established functions in stress response, also contribute to development by an unknown mechanism. We show by genome-wide location analysis that JNK binds to a large set of active promoters during the differentiation of stem cells into neurons. JNK-bound promoters are enriched with binding motifs for the transcription factor NF-Y but not for AP-1. NF-Y occupies these predicted sites, and overexpression of dominant-negative NF-YA reduces the JNK presence on chromatin. We find that histone H3 Ser10 (H3S10) is a substrate for JNK, and JNK-bound promoters are enriched for H3S10 phosphorylation. Inhibition of JNK signaling in post-mitotic neurons reduces phosphorylation at H3S10 and the expression of target genes. These results establish MAP kinase binding and function on chromatin at a novel class of target genes during stem cell differentiation.
Biochemical and biophysical research communications 2011 SEP
Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells.
Abstract
Abstract
The canonical Wnt signaling pathway can determine human bone marrow stromal (mesenchymal) stem cell (hMSC) differentiation fate into osteoblast or adipocyte lineages. However, its downstream targets in MSC are not well characterized. Thus, using DNA microarrays, we compared global gene expression patterns induced by Wnt3a treatment in two hMSC lines: hMSC-LRP5(T253) and hMSC-LRP5(T244) cells carrying known mutations of Wnt co-receptor LRP5 (T253I or T244M) that either enhances or represses canonical Wnt signaling, respectively. Wnt3a treatment of hMSC activated not only canonical Wnt signaling, but also the non-canonical Wnt/JNK pathway through upregulation of several non-canonical Wnt components e.g. naked cuticle 1 homolog (NKD1) and WNT11. Activation of the non-canonical Wnt/JNK pathway by anisomycin enhanced osteoblast differentiation whereas its inhibition by SP600125 enhanced adipocyte differentiation of hMSC. In conclusion, canonical and non-canonical Wnt signaling cooperate in determining MSC differentiation fate.
The Journal of biological chemistry 2010 FEB
Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes.
Abstract
Abstract
Increasing adipocyte size as well as numbers is important in the development of obesity and type 2 diabetes, with adipocytes being generated from mesenchymal precursor cells. This process includes the determination of mesenchymal stem cells (MSC) into preadipocytes (PA) and the differentiation of PA into mature fat cells. Although the process of differentiation has been highly investigated, the determination in humans is poorly understood. In this study, we compared human MSC and human committed PA on a cellular and molecular level to gain further insights into the regulatory mechanisms in the determination process. Both cell types showed similar morphology and expression patterns of common mesenchymal and hematopoietic surface markers. However, although MSC were able to differentiate into adipocytes and osteocytes, PA were only able to undergo adipogenesis, indicating that PA lost their multipotency during determination. WNT-5a expression showed significantly higher levels in MSC compared with PA suggesting that WNT-5a down-regulation might be important in the determination process. Indeed, incubation of human MSC in medium containing neutralizing WNT-5a antibodies abolished their ability to undergo osteogenesis, although adipogenesis was still possible. An opposite effect was achieved using recombinant WNT-5a protein. On a molecular level, WNT-5a was found to promote c-Jun N-terminal kinase-dependent intracellular signaling in MSC. Activation of this noncanonical pathway resulted in the induction of osteopontin expression further indicating pro-osteogenic effects of WNT-5a. Our data suggest that WNT-5a is necessary to maintain osteogenic potential of MSC and that inhibition of WNT-5a signaling therefore plays a role in their determination into PA in humans.
The Journal of cell biology 2009 APR
Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells.
Abstract
Abstract
Genetic evidence indicates that Wnt signaling is critically involved in bone homeostasis. In this study, we investigated the functions of canonical Wnts on differentiation of adult multipotent human mesenchymal stem cells (hMSCs) in vitro and in vivo. We observe differential sensitivities of hMSCs to Wnt inhibition of osteogenesis versus adipogenesis, which favors osteoblastic commitment under binary in vitro differentiation conditions. Wnt inhibition of osteogenesis is associated with decreased expression of osteoblastic transcription factors and inhibition of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation, which are involved in osteogenic differentiation. An hMSC subpopulation exhibits high endogenous Wnt signaling, the inhibition of which enhances osteogenic and adipogenic differentiation in vitro. In an in vivo bone formation model, high levels of Wnt signaling inhibit de novo bone formation by hMSCs. However, hMSCs with exogenous expression of Wnt1 but not stabilized beta-catenin markedly stimulate bone formation by naive hMSCs, arguing for an important role of a canonical Wnt gradient in hMSC osteogenesis in vivo.

 网站首页
网站首页