概要
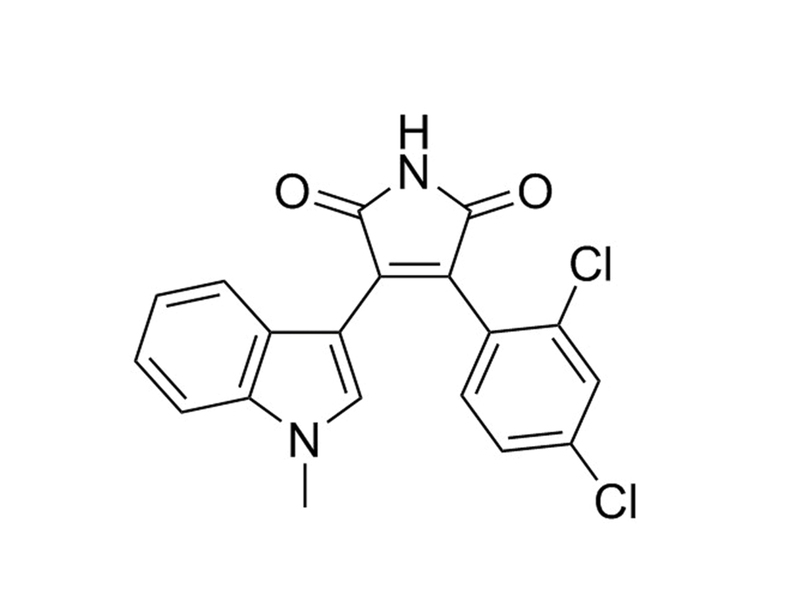
SB216763 is a cell-permeable ATP-competitive inhibitor of glycogen synthase kinase 3α (GSK3α, IC₅₀ = 34 nM) and GSK3β isozymes (Coghlan et al.). GSK3 is a serine/threonine protein kinase that is inhibited by a variety of extracellular stimuli including insulin, growth factors, cell specification factors, and cell adhesion.
MAINTENANCE AND SELF-RENEWAL
· Maintains mouse embryonic stem (ES) cells in an undifferentiated, pluripotent state for up to two months when co-cultured with mouse embryonic fibroblasts (MEFs) in the absence of leukemia inhibitory factor (LIF; Kirby et al.).
· Promotes the proliferation of primary mouse retinal stem cells (Inoue et al.).
· Increases neural progenitor proliferation in mouse brains (Mao et al.).
· Increases symmetric division of neural stem cells (NSCs) in in vivo and in vitro models of the adult mouse brain (Piccin and Morshead).
· Inhibits adipocyte differentiation in human mesenchymal stem cells (MSCs; Shen et al.).
· Promotes the generation of hematopoietic stem cells (HSCs) in aorta-gonad-mesonephros (AGM) explant cultures (Ruiz-Herguido et al.).
DIFFERENTIATION
· Enhances the insulin-induced differentiation of quiescent reserve cells from cultured mouse myoblasts (Rochat et al.).
· Stimulates NSC differentiation in cultured rat neurospheres (Maurer et al.).
· Induces neuronal differentiation in cultured human neural progenitor cells (NPCs; Lange et al.).
· Promotes differentiation of dendritic cells from cultured mouse hematopoietic progenitor cells (HPCs; Zhou et al.).
CANCER RESEARCH
· Induces differentiation and reduces the cancer stem cell population of cultured human glioblastoma cells (Korur et al.).
MAINTENANCE AND SELF-RENEWAL
· Maintains mouse embryonic stem (ES) cells in an undifferentiated, pluripotent state for up to two months when co-cultured with mouse embryonic fibroblasts (MEFs) in the absence of leukemia inhibitory factor (LIF; Kirby et al.).
· Promotes the proliferation of primary mouse retinal stem cells (Inoue et al.).
· Increases neural progenitor proliferation in mouse brains (Mao et al.).
· Increases symmetric division of neural stem cells (NSCs) in in vivo and in vitro models of the adult mouse brain (Piccin and Morshead).
· Inhibits adipocyte differentiation in human mesenchymal stem cells (MSCs; Shen et al.).
· Promotes the generation of hematopoietic stem cells (HSCs) in aorta-gonad-mesonephros (AGM) explant cultures (Ruiz-Herguido et al.).
DIFFERENTIATION
· Enhances the insulin-induced differentiation of quiescent reserve cells from cultured mouse myoblasts (Rochat et al.).
· Stimulates NSC differentiation in cultured rat neurospheres (Maurer et al.).
· Induces neuronal differentiation in cultured human neural progenitor cells (NPCs; Lange et al.).
· Promotes differentiation of dendritic cells from cultured mouse hematopoietic progenitor cells (HPCs; Zhou et al.).
CANCER RESEARCH
· Induces differentiation and reduces the cancer stem cell population of cultured human glioblastoma cells (Korur et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | SB216763 | 72872, 72874 | All | English |
| Safety Data Sheet | SB216763 | 72872, 72874 | All | English |
数据及文献
Publications (12)
The Journal of experimental medicine 2012 JUL
Hematopoietic stem cell development requires transient Wnt/β-catenin activity.
Abstract
Abstract
Understanding how hematopoietic stem cells (HSCs) are generated and the signals that control this process is a crucial issue for regenerative medicine applications that require in vitro production of HSC. HSCs emerge during embryonic life from an endothelial-like cell population that resides in the aorta-gonad-mesonephros (AGM) region. We show here that β-catenin is nuclear and active in few endothelial nonhematopoietic cells closely associated with the emerging hematopoietic clusters of the embryonic aorta during mouse development. Importantly, Wnt/β-catenin activity is transiently required in the AGM to generate long-term HSCs and to produce hematopoietic cells in vitro from AGM endothelial precursors. Genetic deletion of β-catenin from the embryonic endothelium stage (using VE-cadherin-Cre recombinase), but not from embryonic hematopoietic cells (using Vav1-Cre), precludes progression of mutant cells toward the hematopoietic lineage; however, these mutant cells still contribute to the adult endothelium. Together, those findings indicate that Wnt/β-catenin activity is needed for the emergence but not the maintenance of HSCs in mouse embryos.
PloS one 2012 JAN
Glycogen synthase kinase 3 (GSK3) inhibitor, SB-216763, promotes pluripotency in mouse embryonic stem cells.
Abstract
Abstract
Canonical Wnt/β-catenin signaling has been suggested to promote self-renewal of pluripotent mouse and human embryonic stem cells. Here, we show that SB-216763, a glycogen synthase kinase-3 (GSK3) inhibitor, can maintain mouse embryonic stem cells (mESCs) in a pluripotent state in the absence of exogenous leukemia inhibitory factor (LIF) when cultured on mouse embryonic fibroblasts (MEFs). MESCs maintained with SB-216763 for one month were morphologically indistinguishable from LIF-treated mESCs and expressed pluripotent-specific genes Oct4, Sox2, and Nanog. Furthermore, Nanog immunostaining was more homogenous in SB-216763-treated colonies compared to LIF. Embryoid bodies (EBs) prepared from these mESCs expressed early-stage markers for all three germ layers, and could efficiently differentiate into cardiac-like cells and MAP2-immunoreactive neurons. To our knowledge, SB-216763 is the first GSK3 inhibitor that can promote self-renewal of mESC co-cultured with MEFs for more than two months.
Stem cells (Dayton, Ohio) 2011 MAR
Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury.
Abstract
Abstract
Neural stem cells comprise a small population of subependymal cells in the adult brain that divide asymmetrically under baseline conditions to maintain the stem cell pool and divide symmetrically in response to injury to increase their numbers. Using in vivo and in vitro models, we demonstrate that Wnt signaling plays a role in regulating the symmetric divisions of adult neural stem cells with no change in the proliferation kinetics of the progenitor population. Using BAT-gal transgenic reporter mice to identify cells with active Wnt signaling, we demonstrate that Wnt signaling is absent in stem cells in conditions where they are dividing asymmetrically and that it is upregulated when stem cells are dividing symmetrically, such as (a) during subependymal regeneration in vivo, (b) in response to stroke, and (c) during colony formation in vitro. Moreover, we demonstrate that blocking Wnt signaling in conditions where neural stem cells are dividing symmetrically inhibits neural stem cell expansion both in vivo and in vitro. Together, these findings reveal that the mechanism by which Wnt signaling modulates the size of the stem cell pool is by regulating the symmetry of stem cell division.
Neuroscience letters 2011 JAN
Small molecule GSK-3 inhibitors increase neurogenesis of human neural progenitor cells.
Abstract
Abstract
Human neural progenitor cells provide a source for cell replacement therapy to treat neurodegenerative diseases. Therefore, there is great interest in mechanisms and tools to direct the fate of multipotent progenitor cells during their differentiation to increase the yield of a desired cell type. We tested small molecule inhibitors of glycogen synthase kinase-3 (GSK-3) for their functionality and their influence on neurogenesis using the human neural progenitor cell line ReNcell VM. Here we report the enhancement of neurogenesis of human neural progenitor cells by treatment with GSK-3 inhibitors. We tested different small molecule inhibitors of GSK-3 i.e. LiCl, sodium-valproate, kenpaullone, indirubin-3-monoxime and SB-216763 for their ability to inhibit GSK-3 in human neural progenitor cells. The highest in situ GSK-3 inhibitory effect of the drugs was found for kenpaullone and SB-216763. Accordingly, kenpaullone and SB-216763 were the only drugs tested in this study to stimulate the Wnt/β-catenin pathway that is antagonized by GSK-3. Analysis of human neural progenitor differentiation revealed an augmentation of neurogenesis by SB-216763 and kenpaullone, without changing cell cycle exit or cell survival. Small molecule inhibitors of GSK-3 enhance neurogenesis of human neural progenitor cells and may be used to direct the differentiation of neural stem and progenitor cells in therapeutic applications.
Experimental cell research 2011 AUG
Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells.
Abstract
Abstract
The WNT signaling pathway plays important roles in the self-renewal and differentiation of mesenchymal stem cells (MSCs). Little is known about WNT signaling in adipocyte differentiation of human MSCs. In this study, we tested the hypothesis that canonical and non-canonical WNTs differentially regulate in vitro adipocytogenesis in human MSCs. The expression of adipocyte gene PPARγ2, lipoprotein lipase, and adipsin increased during adipocytogenesis of hMSCs. Simultaneously, the expression of canonical WNT2, 10B, 13, and 14 decreased, whereas non-canonical WNT4 and 11 increased, and WNT5A was unchanged. A small molecule WNT mimetic, SB-216763, increased accumulation of β-catenin protein, inhibited induction of WNT4 and 11 and inhibited adipocytogenesis. In contrast, knockdown of β-catenin with siRNA resulted in spontaneous adipocytogenesis. These findings support the view that canonical WNT signaling inhibits and non-canonical WNT signaling promotes adipocytogenesis in adult human marrow-derived mesenchymal stem cells.
Cell 2009 MAR
Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling.
Abstract
Abstract
The Disrupted in Schizophrenia 1 (DISC1) gene is disrupted by a balanced chromosomal translocation (1; 11) (q42; q14.3) in a Scottish family with a high incidence of major depression, schizophrenia, and bipolar disorder. Subsequent studies provided indications that DISC1 plays a role in brain development. Here, we demonstrate that suppression of DISC1 expression reduces neural progenitor proliferation, leading to premature cell cycle exit and differentiation. Several lines of evidence suggest that DISC1 mediates this function by regulating GSK3beta. First, DISC1 inhibits GSK3beta activity through direct physical interaction, which reduces beta-catenin phosphorylation and stabilizes beta-catenin. Importantly, expression of stabilized beta-catenin overrides the impairment of progenitor proliferation caused by DISC1 loss of function. Furthermore, GSK3 inhibitors normalize progenitor proliferation and behavioral defects caused by DISC1 loss of function. Together, these results implicate DISC1 in GSK3beta/beta-catenin signaling pathways and provide a framework for understanding how alterations in this pathway may contribute to the etiology of psychiatric disorders.

 网站首页
网站首页