概要
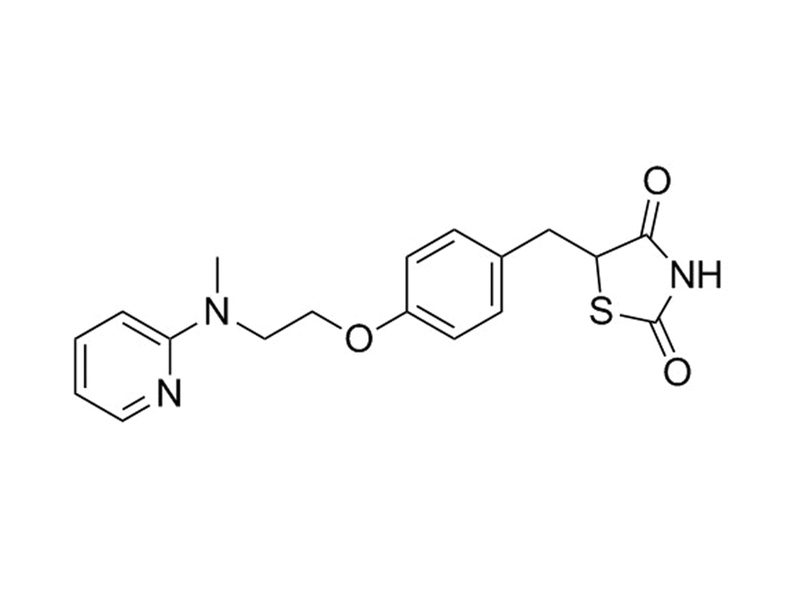
Rosiglitazone is a potent and selective PPARγ ligand. It binds to the PPARγ ligand-binding domain with a Kd of 43 nM (Lehmann et al.). It activates luciferase-based expression constructs PPARγ1 and PPARγ2 with EC₅₀ values of approximately 30 nM and 100 nM, respectively (Lehmann et al.).
MAINTENANCE AND SELF-RENEWAL
· Stimulates embryonic mouse neural stem cell proliferation and inhibits neuronal differentiation (Wada et al.).
DIFFERENTIATION
· Induces adipocyte differentiation in C3H10T1/2 stem cells (Lehmann et al.).
· Promotes endothelial differentiation, and inhibits smooth muscle differentiation, in angiogenic progenitor cells (Wang et al.).
· Stimulates adipocyte differentiation, and decreases osteogenesis, in mouse and human bone marrow-derived mesenchymal stem cells (Ali et al.; Benvenuti et al.; Lecka-Czernik et al.; Sorocéanu et al.).
· Enhances osteoclast formation in mouse bone marrow cells stimulated with RANKL and M-CSF (Wu et al.).
MAINTENANCE AND SELF-RENEWAL
· Stimulates embryonic mouse neural stem cell proliferation and inhibits neuronal differentiation (Wada et al.).
DIFFERENTIATION
· Induces adipocyte differentiation in C3H10T1/2 stem cells (Lehmann et al.).
· Promotes endothelial differentiation, and inhibits smooth muscle differentiation, in angiogenic progenitor cells (Wang et al.).
· Stimulates adipocyte differentiation, and decreases osteogenesis, in mouse and human bone marrow-derived mesenchymal stem cells (Ali et al.; Benvenuti et al.; Lecka-Czernik et al.; Sorocéanu et al.).
· Enhances osteoclast formation in mouse bone marrow cells stimulated with RANKL and M-CSF (Wu et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Rosiglitazone | 72622, 72624 | All | English |
| Safety Data Sheet | Rosiglitazone | 72622, 72624 | All | English |
数据及文献
Publications (8)
Journal of cellular biochemistry 2013 SEP
Regulation of selective PPARγ modulators in the differentiation of osteoclasts.
Abstract
Abstract
Diabetes is the most common chronic disease in the world and causes complications with many diseases, such as heart disease and osteoporosis. Osteoporosis is a systemic bone disease characterized by imbalance in bone resorption and bone formation. Osteoclast is type of bone cell that functions in bone resorption and plays a critical role in bone remodeling. Rosiglitazone and pioglitazone, which belong to Thiazolidinediones(TZDs), are commonly used antidiabetic drugs. As PPARγ full agonists, they can activate PPARγ in a ligand-dependent way. Recent studies indicate that these PPARγ full agonists have some side effects, such as weight gain and bone loss, which may increase the risk of osteoporosis. In contrast, selective PPARγ Modulators (SPPARγMs) are novel PPARγ ligands that can activate PPARγ in different ways and lead to distinct downstream genes. Mice bone marrow cells were stimulated with recombinant mouse RANKL and M-CSF to generate osteoclasts. To determine the effect on osteoclasts formation, PPARγ ligands (Rosiglitazone, Fmoc-L-Leu, and Telmisartan) were added at the beginning of the culture. Rosiglitazone significantly increased the differentiation of multinucleated osteoclasts, while osteoclasts formation triggered by SPPARγMs was much less than that displayed by rosiglitazone. We found that the enhancement of PPARγ ligands may be associated with TRAF6 and downstream ERK signal pathway. We also demonstrated osteoclasts show characteristic M2 phenotype and can be further promoted by PPARγ ligands, especially rosiglitazone. In conclusion, reduced osteoclasts differentiation characteristic of SPPARγMs highlights SPPARγMs potential as therapeutic targets in diabetes, versus traditional antidiabetic drugs.
Journal of endocrinological investigation 2007 OCT
Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells.
Abstract
Abstract
Thiazolidinediones (TZD) are widely prescribed for the treatment of Type 2 diabetes. Increased loss of bone mass and a higher incidence of fractures have been associated with the use of this class of drugs in post-menopausal women. In vitro studies performed in rodent cell models indicated that rosiglitazone (RGZ), one of the TZD, inhibited osteoblastogenesis and induced adipogenesis in bone marrow progenitor cells. The objective of the present study was to determine for the first time the RGZ-dependent shift from osteoblastogenesis toward adipogenesis using a human cell model. To this purpose, bone marrow-derived mesenchymal stem cells were characterized and induced to differentiate along osteogenic and adipogenic lineages. We found that the exposure to RGZ potentiated adipogenic differentiation and shifted the differentiation toward an osteogenic phenotype into an adipogenic phenotype, as assessed by the appearance of lipid droplets. Accordingly, RGZ markedly increased the expression of the typical marker of adipogenesis fatty-acid binding protein 4, whereas it reduced the expression of Runx2, a marker of osteoblastogenesis. This is the first demonstration that RGZ counteracts osteoblastogenesis and induces a preferential differentiation into adipocytes in human mesenchymal stem cells.
The Journal of biological chemistry 2006 MAY
Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation.
Abstract
Abstract
Peroxisome proliferator-activated receptor gamma (PPARgamma) plays an important role in insulin sensitivity, tissue homeostasis, and regulating cellular functions. We found high-level expression of PPARgamma in embryo mouse brain and neural stem cells (NSCs), in contrast to extremely low levels in adult mouse brain. Here, we show that PPARgamma mediates the proliferation and differentiation of murine NSCs via up-regulation of the epidermal growth factor receptor and activation of the ERK pathway. Cell growth rates of NSCs prepared from heterozygous PPARgamma-deficient mouse brains, PPARgamma-RNA-silenced NSCs, and PPARgamma dominant-negative NSCs were significantly decreased compared with those of wild-type NSCs. Physiological concentrations of PPARgamma agonists, rosiglitazone and pioglitazone, stimulated NSC growth, whereas antagonists caused cell death in a concentration-dependent manner via activation of the caspase cascade. The stimulation of cell growth by PPARgamma was associated with a rapid activation of the ERK pathway by phosphorylation and up-regulation of epidermal growth factor receptor and cyclin B protein levels. In contrast, activation of PPARgamma by agonists inhibited the differentiation of NSCs into neurons. The inhibition of differentiation was associated with an activation of STAT3. These data indicate that PPARgamma regulates the development of the central nervous system during early embryogenesis via control of NSC proliferation.
Endocrinology 2005 MAR
Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation.
Abstract
Abstract
Because osteoblasts and marrow adipocytes are derived from a common mesenchymal progenitor, increased adipogenesis may occur at the expense of osteoblasts, leading to bone loss. Our previous in vitro studies indicated that activation of the proadipogenic transcription factor peroxisome proliferator-activated receptor isoform gamma 2 with rosiglitazone suppressed osteoblast differentiation. Here, we show that 5-month-old Swiss-Webster mice receiving rosiglitazone for 28 d exhibited bone loss associated with an increase in marrow adipocytes, a decrease in the ratio of osteoblasts to osteoclasts, a reduction in bone formation rate, and a reduction in wall width--an index of the amount of bone formed by each team of osteoblasts. Rosiglitazone had no effect on the number of early osteoblast or osteoclast progenitors, or on osteoblast life span, but decreased the expression of the key osteoblastogenic transcription factors Runx2 and Osterix in cultures of marrow-derived mesenchymal progenitors. These effects were associated with diversion of bipotential progenitors from the osteoblast to the adipocyte lineage, and suppression of the differentiation of monopotential osteoblast progenitors. However, rosiglitazone had no effect on osteoblastic cells at later stages of differentiation. Hence, rosiglitazone attenuates osteoblast differentiation and thereby reduces bone formation rate in vivo, leading to bone loss. These findings provide a mechanistic explanation for the recent evidence that peroxisome proliferator-activated receptor isoform gamma activation is a negative regulator of bone mass and suggest that the increased production of oxidized fatty acids with age may indeed be an important mechanism for age-related osteoporosis in humans.
The Journal of endocrinology 2004 OCT
Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis.
Abstract
Abstract
Thiazolidinediones (TZDs) increase peripheral tissue insulin sensitivity in patients with type 2 diabetes mellitus by activating the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARgamma). In bone marrow stromal cell cultures and in vivo, activation of PPARgamma by high doses (20 mg/kg/day) of TZDs has been reported to alter stem cell differentiation by promoting commitment of progenitor cells to the adipocytic lineage while inhibiting osteoblastogenesis. Here, we have examined the in vivo effects of low-dose rosiglitazone (3 mg/kg/day) on bone, administered to mice by gavage for 90 days. Rosiglitazone-treated mice had increased weight when compared with controls, with no significant alterations in serum levels of glucose, calcium or parathyroid hormone (PTH). Bone mineral density (BMD) at the lumbar vertebrae (L1-L4), ilium/sacrum, and total body was diminished by rosiglitazone treatment. Histologically, bone was characterized by decreased trabecular bone volume and increased marrow space with no significant change in bone marrow adipocity. Decreased osteoblast number and activity due to increased apoptotic death of osteoblasts and osteocytes was apparent while osteoclast parameters and serum levels of osteocalcin, alkaline phosphatase activity, and leptin were unaltered by rosiglitazone treatment. Therefore, the imbalance in bone remodeling that follows rosiglitazone administration arises from increased apoptotic death of osteogenic cells and diminished bone formation leading to the observed decrease in trabecular bone volume and BMD. These novel in vivo effects of TZDs on bone are of clinical relevance as patients with type 2 diabetes mellitus and other insulin resistant states treated with these agents may potentially be at increased risk of osteoporosis.
Circulation 2004 MAR
Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy.
Abstract
Abstract
BACKGROUND: Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists inhibit vascular smooth muscle proliferation and migration and improve endothelial function. It is unknown whether PPAR-gamma agonists favorably modulate bone marrow (BM)-derived angiogenic progenitor cells (APCs) to promote endothelial lineage differentiation and early reendothelialization after vascular intervention. METHODS AND RESULTS: C57/BL6 mice, treated with or without rosiglitazone (8 mg/kg per day), a PPAR-gamma agonist, underwent femoral angioplasty. Rosiglitazone treatment attenuated neointimal formation (intima/media ratio: 0.98+/-0.12 [rosiglitazone] versus 3.1+/-0.5 [control]; Ptextless0.001; n=10 per group). Using a BM transplantation model, we identified that 58+/-12% of the cells within the neointima at 4 weeks were derived from the BM. Pure endothelial marker-positive, pure alpha-smooth muscle actin (alphaSMA)-positive, or double-positive APCs could be found both in mouse BM and in human peripheral blood after culture in conditional medium enriched with vascular endothelial growth factor. Rosiglitazone caused a 6-fold (Ptextless0.001) increase in colony formation by human endothelial progenitor cells, promoted the differentiation of APCs toward the endothelial lineage in mouse BM in vivo (0.66+/-0.06% [control] to 0.95+/-0.08% [rosiglitazone]; Ptextless0.05) and in human peripheral blood in vitro (13.2+/-1.5% [control] to 28.4+/-3.3% [rosiglitazone]; Ptextless0.05), and inhibited the differentiation toward the smooth muscle cell lineage. Within the neointima, rosiglitazone also stimulated APCs to differentiate into mature endothelial cells and caused earlier reendothelialization compared with controls (31+/-5 versus 8+/-2 CD31-positive cells per millimeter of neointimal surface on day 14; Ptextless0.01). CONCLUSIONS: Similar to embryonic stem cell-derived progenitors, the adult BM and peripheral blood harbor APCs that are at least bipotential and able to differentiate into endothelial and smooth muscle lineages. The PPAR-gamma agonist rosiglitazone promotes the differentiation of these APCs toward the endothelial lineage and attenuates restenosis after angioplasty.

 网站首页
网站首页