概要
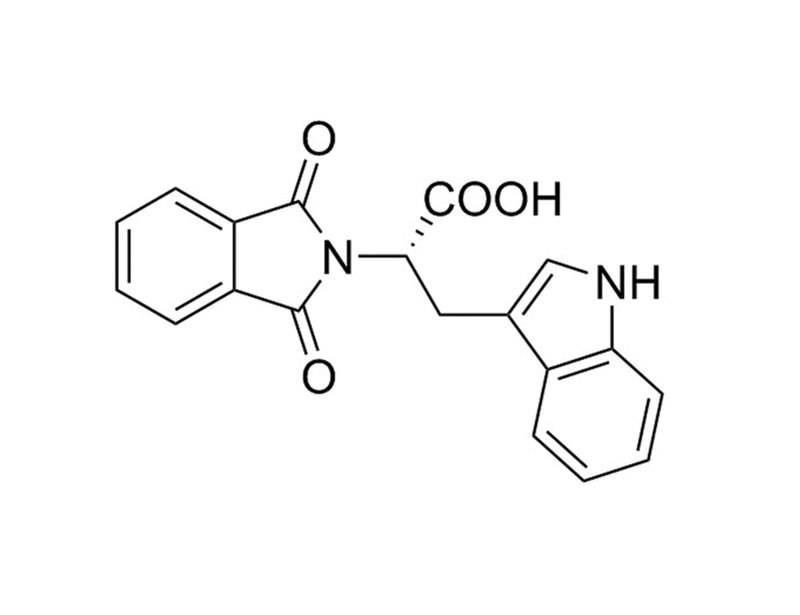
RG108 is an epigenetic modifier that inhibits DNA methyltransferase (IC₅₀ = 115 nM). RG108 is a non-nucleoside inhibitor that acts by direct binding to the methyltransferase enzyme whereby it blocks the enzyme active site. (Brueckner et al., Stresemann et al.)
REPROGRAMMING
· Enhances reprogramming efficiency of human and mouse somatic cells to induced pluripotent stem (iPS) cells (Mali et al., Pasha et al., Shi et al.).
REPROGRAMMING
· Enhances reprogramming efficiency of human and mouse somatic cells to induced pluripotent stem (iPS) cells (Mali et al., Pasha et al., Shi et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RG108 | 72212, 72214 | All | English |
| Safety Data Sheet | RG108 | 72212, 72214 | All | English |
数据及文献
Publications (5)
PloS one 2011 JAN
Efficient non-viral reprogramming of myoblasts to stemness with a single small molecule to generate cardiac progenitor cells.
Abstract
Abstract
UNLABELLED The current protocols for generation of induced pluripotent stem (iPS) cells involve genome integrating viral vectors which may induce tumorgenesis. The aim of this study was to develop and optimize a non-viral method without genetic manipulation for reprogramming of skeletal myoblasts (SMs) using small molecules. METHODS AND RESULTS SMs from young male Oct3/4-GFP(+) transgenic mouse were treated with DNA methyltransferase (DNMT) inhibitor, RG108. Two weeks later, GFP(+) colonies of SM derived iPS cells (SiPS) expressing GFP and with morphological similarity of mouse embryonic stem (ESCs) were formed and propagated in vitro. SiPS were positive for alkaline phosphatase activity, expressed SSEA1, displayed ES cell specific pluripotency markers and formed teratoma in nude mice. Optimization of culture conditions for embryoid body (EBs) formation yielded spontaneously contracting EBs having morphological, molecular, and ultra-structural similarities with cardiomyocytes and expressed early and late cardiac markers. miR profiling showed abrogation of let-7 family and upregulation of ESCs specific miR-290-295 cluster thus indicating that SiPS were similar to ESCs in miR profile. Four weeks after transplantation into the immunocompetent mice model of acute myocardial infarction (n = 12 per group), extensive myogenesis was observed in SiPS transplanted hearts as compared to DMEM controls (n = 6 per group). A significant reduction in fibrosis and improvement in global heart function in the hearts transplanted with SiPS derived cardiac progenitor cells were observed. CONCLUSIONS Reprogramming of SMs by DNMT inhibitor is a simple, reproducible and efficient technique more likely to generate transgene integration-free iPS cells. Cardiac progenitors derived from iPS cells propagated extensively in the infarcted myocardium without tumorgenesis and improved cardiac function.
Stem cells (Dayton, Ohio) 2010 APR
Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes.
Abstract
Abstract
We report here that butyrate, a naturally occurring fatty acid commonly used as a nutritional supplement and differentiation agent, greatly enhances the efficiency of induced pluripotent stem (iPS) cell derivation from human adult or fetal fibroblasts. After transient butyrate treatment, the iPS cell derivation efficiency is enhanced by 15- to 51-fold using either retroviral or piggyBac transposon vectors expressing 4 to 5 reprogramming genes. Butyrate stimulation is more remarkable (textgreater100- to 200-fold) on reprogramming in the absence of either KLF4 or MYC transgene. Butyrate treatment did not negatively affect properties of iPS cell lines established by either 3 or 4 retroviral vectors or a single piggyBac DNA transposon vector. These characterized iPS cell lines, including those derived from an adult patient with sickle cell disease by either the piggyBac or retroviral vectors, show normal karyotypes and pluripotency. To gain insights into the underlying mechanisms of butyrate stimulation, we conducted genome-wide gene expression and promoter DNA methylation microarrays and other epigenetic analyses on established iPS cells and cells from intermediate stages of the reprogramming process. By days 6 to 12 during reprogramming, butyrate treatment enhanced histone H3 acetylation, promoter DNA demethylation, and the expression of endogenous pluripotency-associated genes, including DPPA2, whose overexpression partially substitutes for butyrate stimulation. Thus, butyrate as a cell permeable small molecule provides a simple tool to further investigate molecular mechanisms of cellular reprogramming. Moreover, butyrate stimulation provides an efficient method for reprogramming various human adult somatic cells, including cells from patients that are more refractory to reprogramming.
Cell stem cell 2008 NOV
Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds.
Abstract
Abstract
Somatic cells can be induced into pluripotent stem cells (iPSCs) with a combination of four transcription factors, Oct4/Sox2/Klf4/c-Myc or Oct4/Sox2/Nanog/LIN28. This provides an enabling platform to obtain patient-specific cells for various therapeutic and research applications. However, several problems remain for this approach to be therapeutically relevant due to drawbacks associated with efficiency and viral genome integration. Recently, it was shown that neural progenitor cells (NPCs) transduced with Oct4/Klf4 can be reprogrammed into iPSCs. However, NPCs express Sox2 endogenously, possibly facilitating reprogramming in the absence of exogenous Sox2. In this study, we identified a small-molecule combination, BIX-01294 and BayK8644, that enables reprogramming of Oct4/Klf4-transduced mouse embryonic fibroblasts, which do not endogenously express the factors essential for reprogramming. This study demonstrates that small molecules identified through a phenotypic screen can compensate for viral transduction of critical factors, such as Sox2, and improve reprogramming efficiency.
Cancer research 2006 MAR
Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines.
Abstract
Abstract
DNA methyltransferase inhibitors represent promising new drugs for cancer therapies. The first of these compounds (5-azacytidine, Vidaza) has recently been approved as an antitumor agent, and others are presently in various stages of their preclinical or clinical development. Most of the archetypal inhibitors have been established and characterized in different experimental systems, which has thus far precluded their direct comparison. We have now established defined experimental conditions that allowed a comparative analysis of the six most widely known DNA methyltransferase inhibitors: 5-azacytidine (5-aza-CR), 5-aza-2'-deoxycytidine (5-aza-CdR), zebularine, procaine, (-)-epigallocatechin-3-gallate (EGCG), and RG108. Of these, 5-aza-CR, 5-aza-CdR, zebularine, and EGCG were found to exhibit significant cytotoxicity in human cancer cell lines. 5-aza-CdR and EGCG were also found to be genotoxic, as evidenced by the induction of micronuclei. In addition, 5-aza-CR, 5-aza-CdR, zebularine, and RG108 caused concentration-dependent demethylation of genomic DNA, whereas procaine and EGCG failed to induce significant effects. Finally, the experiments in cancer cell lines were complemented by a cell-free in vitro assay with purified recombinant DNA methyltransferase, which indicated that RG108 is the only drug capable of direct enzyme inhibition. These results show a substantial diversity in the molecular activities of DNA methyltransferase inhibitors and provide valuable insights into the developmental potential of individual drugs.
Cancer research 2005 JUL
Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases.
Abstract
Abstract
DNA methylation regulates gene expression in normal and malignant cells. The possibility to reactivate epigenetically silenced genes has generated considerable interest in the development of DNA methyltransferase inhibitors. Here, we provide a detailed characterization of RG108, a novel small molecule that effectively blocked DNA methyltransferases in vitro and did not cause covalent enzyme trapping in human cell lines. Incubation of cells with low micromolar concentrations of the compound resulted in significant demethylation of genomic DNA without any detectable toxicity. Intriguingly, RG108 caused demethylation and reactivation of tumor suppressor genes, but it did not affect the methylation of centromeric satellite sequences. These results establish RG108 as a DNA methyltransferase inhibitor with fundamentally novel characteristics that will be particularly useful for the experimental modulation of epigenetic gene regulation.

 网站首页
网站首页