概要
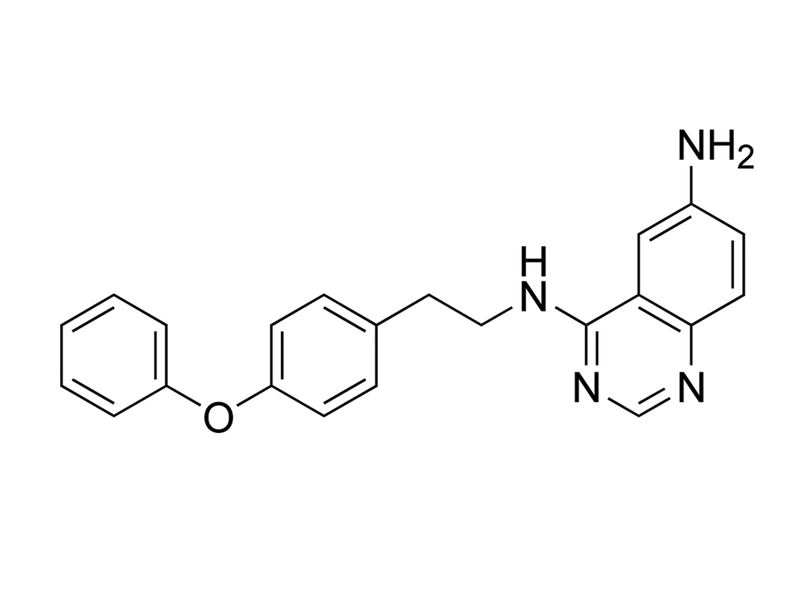
QNZ is a quinazoline derivative that inhibits nuclear factor (NF)-κB activation (IC₅₀ = 11 nM in human Jurkat T lymphocyte cells). NF-κB enhances the transcription of pro-inflammatory cytokines, and QNZ inhibits lipopolysaccharide (LPS)-stimulated tumor necrosis factor (TNF)-α production in mouse splenocytes (IC₅₀ = 7 nM;Tobe et al.), as well as CXCL1-mediated pro-inflammatory increase in potassium currents in adult rat neurons (Yang et al). It does not inhibit kinases in a standard screen (Wu et al.).
MAINTENANCE
· Neuroprotective in a glutamate toxicity assay using YAC128 medium spiny neuron cultures (Wu et al.).
DISEASE MODELING
· Blocks amyloid precursor protein release in human SH-SY5Y neuroblastoma cells caused by muscarinic receptor activation (Choi et al.).
MAINTENANCE
· Neuroprotective in a glutamate toxicity assay using YAC128 medium spiny neuron cultures (Wu et al.).
DISEASE MODELING
· Blocks amyloid precursor protein release in human SH-SY5Y neuroblastoma cells caused by muscarinic receptor activation (Choi et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | QNZ | 73352 | All | English |
| Safety Data Sheet | QNZ | 73352 | All | English |
数据及文献
Publications (4)
Chemistry & biology 2011
Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington's disease treatment.
Abstract
Abstract
Huntington's disease (HD) is a neurodegenerative disorder caused by a polyglutamine expansion within Huntingtin (Htt) protein. In the phenotypic screen we identified a class of quinazoline-derived compounds that delayed a progression of a motor phenotype in transgenic Drosophila HD flies. We found that the store-operated calcium (Ca(2+)) entry (SOC) pathway activity is enhanced in neuronal cells expressing mutant Htt and that the identified compounds inhibit SOC pathway in HD neurons. The same compounds exerted neuroprotective effects in glutamate-toxicity assays with YAC128 medium spiny neurons primary cultures. We demonstrated a key role of TRPC1 channels in supporting SOC pathway in HD neurons. We concluded that the TRPC1-mediated neuronal SOC pathway constitutes a novel target for HD treatment and that the identified compounds represent a novel class of therapeutic agents for treatment of HD and possibly other neurodegenerative disorders.
Molecular pain 2009 JAN
NF-kappaB mediated enhancement of potassium currents by the chemokine CXCL1/growth related oncogene in small diameter rat sensory neurons.
Abstract
Abstract
BACKGROUND: Inflammatory processes play important roles in both neuropathic and inflammatory pain states, but the effects of inflammation per se within the sensory ganglia are not well understood. The cytokine growth-related oncogene (GRO/KC; CXCL1) shows strong, rapid upregulation in dorsal root ganglion (DRG) in both nerve injury and inflammatory pain models. We examined the direct effects of GRO/KC on small diameter DRG neurons, which are predominantly nociceptive. Whole cell voltage clamp technique was used to measure voltage-activated potassium (K) currents in acutely cultured adult rat small diameter sensory neurons. Fluorescently labeled isolectin B4 (IB4) was used to classify cells as IB4-positive or IB4-negative. RESULTS: In IB4-negative neurons, voltage-activated K current densities of both transient and sustained components were increased after overnight incubation with GRO/KC (1.5 nM), without marked changes in voltage dependence or kinetics. The average values for the slow and fast decay time constants at 20 mV were unchanged by GRO/KC. The amplitude of the fast inactivating component increased significantly with no large shifts in the voltage dependence of inactivation. The increase in K currents was completely blocked by co-incubation with protein synthesis inhibitor cycloheximide (CHX) or NF-kappaB inhibitors pyrrolidine dithiocarbamate (PDTC) or quinazoline (6-Amino-4-(4-phenoxypheny lethylamino;QNZ). In contrast, the voltage-activated K current of IB4-positive neurons was unchanged by GRO/KC. GRO/KC incubation caused no significant changes in the expression level of eight selected voltage-gated K channel genes in quantitative PCR analysis. CONCLUSION: The results suggest that GRO/KC has important effects in inflammatory processes via its direct actions on sensory neurons, and that activation of NF-kappaB is involved in the GRO/KC-induced enhancement of K currents.
The Journal of biological chemistry 2006
Nuclear factor-kappaB activated by capacitative Ca2+ entry enhances muscarinic receptor-mediated soluble amyloid precursor protein (sAPPalpha) release in SH-SY5Y cells.
Abstract
Abstract
G(q/11) protein-coupled muscarinic receptors are known to regulate the release of soluble amyloid precursor protein (sAPPalpha) produced by alpha-secretase processing; however, their signaling mechanisms remain to be elucidated. It has been reported that a muscarinic agonist activates nuclear factor (NF)-kappaB, a transcription factor that has been shown to play an important role in the Alzheimer disease brain, and that NF-kappaB activation is regulated by intracellular Ca2+ level. In the present study, we investigated whether NF-kappaB activation plays a role in muscarinic receptor-mediated sAPPalpha release enhancement and contributes to a changed capacitative Ca2+ entry (CCE), which was suggested to be involved in the muscarinic receptor-mediated stimulation of sAPPalpha release. Muscarinic receptor-mediated NF-kappaB activation was confirmed by observing the translocation of the active subunit (p65) of NF-kappaB to the nucleus by the muscarinic agonist, oxotremorine M (oxoM), in SH-SY5Y neuroblastoma cells expressing muscarinic receptors that are predominantly of the M3 subtype. NF-kappaB activation and sAPPalpha release enhancement induced by oxoM were inhibited by NF-kappaB inhibitors, such as an NF-kappaB peptide inhibitor (SN50), an IkappaB alpha kinase inhibitor (BAY11-7085), a proteasome inhibitor (MG132), the inhibitor of proteasome activity and IkappaB phosphorylation, pyrrolidine dithiocarbamate, the novel NF-kappaB activation inhibitor (6-amino-4-(4-phenoxyphenylethylamino) quinazoline), and by an intracellular Ca2+ chelator (TMB-8). Furthermore, both oxoM-induced NF-kappaB activation and sAPPalpha release were antagonized by CCE inhibitors (gadolinium or SKF96365) but not by voltage-gated Ca2+-channel blockers. On the other hand, treatment of cells with NF-kappaB inhibitors (SN50, BAY11-7085, MG132, or pyrrolidine dithiocarbamate) did not inhibit muscarinic receptor-mediated CCE. These findings provide evidence for the involvement of NF-kappaB regulated by CCE in muscarinic receptor-mediated sAPPalpha release enhancement.
Bioorganic & medicinal chemistry 2003
Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation.
Abstract
Abstract
We disclose here a new structural class of low-molecular-weight inhibitors of NF-kappa B activation that were designed and synthesized by starting from quinazoline derivative 6a. Structure-activity relationship (SAR) studies based on 6a elucidated the structural requirements essential for the inhibitory activity toward NF-kappa B transcriptional activation, and led to the identification of the 6-amino-4-phenethylaminoquinazoline skeleton as the basic framework. In this series of compounds, 11q, containing the 4-phenoxyphenethyl moiety at the C(4)-position, showed strong inhibitory effects on both NF-kappa B transcriptional activation and TNF-alpha production. Furthermore, 11q exhibited an anti-inflammatory effect on carrageenin-induced paw edema in rats.

 网站首页
网站首页