概要
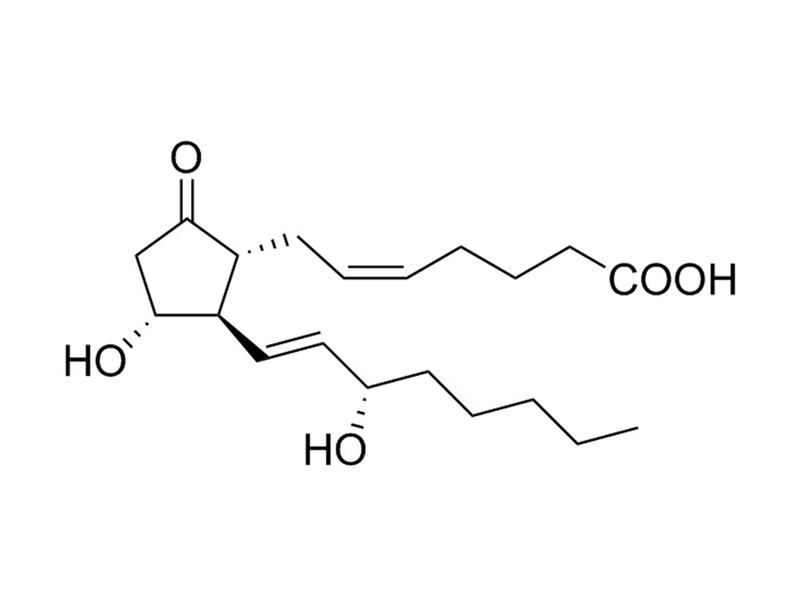
Prostaglandin E₂ (PGE₂) is the most biologically active and well-studied prostaglandin. It binds with very high affinity to the EP1, EP2, EP3, and EP4 receptors (Ki = 9.1, 4.9, 0.33, 0.79 nM respectively). (Abramovitz et al., Bos et al.)
MAINTENANCE AND SELF-RENEWAL
· Required for the development of hematopoietic stem cells (HSCs) in mice and zebrafish (North et al.).
· Improves engraftment of mouse HSCs, possibly through increasing homing, survival, and/or self-renewal (Hoggatt et al. 2009, Hoggatt et al. 2013, North et al.).
DIFFERENTIATION
· Promotes differentiation of hematopoietic progenitor cells from mouse, macaque, and human embryonic stem cells (Gori et al., North et al., Woods et al.).
· Promotes differentiation of myeloid-derived suppressor cells from hematopoietic progenitors (Sinha et al.).
· Promotes differentiation of Th17 cells from naïve T-cells (Boniface et al.).
MAINTENANCE AND SELF-RENEWAL
· Required for the development of hematopoietic stem cells (HSCs) in mice and zebrafish (North et al.).
· Improves engraftment of mouse HSCs, possibly through increasing homing, survival, and/or self-renewal (Hoggatt et al. 2009, Hoggatt et al. 2013, North et al.).
DIFFERENTIATION
· Promotes differentiation of hematopoietic progenitor cells from mouse, macaque, and human embryonic stem cells (Gori et al., North et al., Woods et al.).
· Promotes differentiation of myeloid-derived suppressor cells from hematopoietic progenitors (Sinha et al.).
· Promotes differentiation of Th17 cells from naïve T-cells (Boniface et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Prostaglandin E2 | 72192, 72194 | All | English |
| Safety Data Sheet | Prostaglandin E2 | 72192, 72194 | All | English |
数据及文献
Publications (10)
Cell systems 2020 mar
Gut-Liver Physiomimetics Reveal Paradoxical Modulation of IBD-Related Inflammation by Short-Chain Fatty Acids.
Abstract
Abstract
Although the association between the microbiome and IBD and liver diseases is known, the cause and effect remain elusive. By connecting human microphysiological systems of the gut, liver, and circulating Treg and Th17 cells, we created a multi-organ model of ulcerative colitis (UC) ex vivo. The approach shows microbiome-derived short-chain fatty acids (SCFAs) to either improve or worsen UC severity, depending on the involvement of effector CD4 T cells. Using multiomics, we found SCFAs increased production of ketone bodies, glycolysis, and lipogenesis, while markedly reducing innate immune activation of the UC gut. However, during acute T cell-mediated inflammation, SCFAs exacerbated CD4+ T cell-effector function, partially through metabolic reprograming, leading to gut barrier disruption and hepatic injury. These paradoxical findings underscore the emerging utility of human physiomimetic technology in combination with systems immunology to study causality and the fundamental entanglement of immunity, metabolism, and tissue homeostasis.
Blood 2013 OCT
Prostaglandin E2 enhances long-term repopulation but does not permanently alter inherent stem cell competitiveness.
Abstract
Abstract
Hematopoietic stem cell (HSC) transplantation is a lifesaving therapy for malignant and nonmalignant hematologic diseases and metabolic disorders. Although successful, hematopoietic transplantation can be hindered by inadequate stem cell number or poor engrafting efficiency. To overcome these deficits, we and others have previously reported the HSC-enhancing ability of a short-term exposure of prostaglandin E2 (PGE2); this strategy has now progressed to phase 1 clinical trials in double cord blood transplantation. To further analyze the short- and long-term effects of HSC exposure to PGE2, we followed the repopulation kinetics of PGE2-treated hematopoietic grafts through 5 serial transplantations and compared inherent long-term competitiveness in a HSC head-to-head secondary transplantation model. Treatment with PGE2 did not result in a long-term increase in HSC competitiveness, lineage bias, or enhanced proliferative potential, demonstrating that pulse exposure to PGE2 results in transient increases in HSC homing and engraftment potential.
Blood 2012 SEP
Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells.
Abstract
Abstract
Induced pluripotent stem cell (iPSC) therapeutics are a promising treatment for genetic and infectious diseases. To assess engraftment, risk of neoplastic formation, and therapeutic benefit in an autologous setting, testing iPSC therapeutics in an appropriate model, such as the pigtail macaque (Macaca nemestrina; Mn), is crucial. Here, we developed a chemically defined, scalable, and reproducible specification protocol with bone morphogenetic protein 4, prostaglandin-E2 (PGE2), and StemRegenin 1 (SR1) for hematopoietic differentiation of Mn iPSCs. Sequential coculture with bone morphogenetic protein 4, PGE2, and SR1 led to robust Mn iPSC hematopoietic progenitor cell formation. The combination of PGE2 and SR1 increased CD34(+)CD38(-)Thy1(+)CD45RA(-)CD49f(+) cell yield by 6-fold. CD34(+)CD38(-)Thy1(+)CD45RA(-)CD49f(+) cells isolated on the basis of CD34 expression and cultured in SR1 expanded 3-fold and maintained this long-term repopulating HSC phenotype. Purified CD34(high) cells exhibited 4-fold greater hematopoietic colony-forming potential compared with unsorted hematopoietic progenitors and had bilineage differentiation potential. On the basis of these studies, we calculated the cell yields that must be achieved at each stage to meet a threshold CD34(+) cell dose that is required for engraftment in the pigtail macaque. Our protocol will support scale-up and testing of iPSC-derived CD34(high) cell therapies in a clinically relevant nonhuman primate model.
Stem cells (Dayton, Ohio) 2011 JUL
Brief report: efficient generation of hematopoietic precursors and progenitors from human pluripotent stem cell lines.
Abstract
Abstract
By mimicking embryonic development of the hematopoietic system, we have developed an optimized in vitro differentiation protocol for the generation of precursors of hematopoietic lineages and primitive hematopoietic cells from human embryonic stem cells (ESC) and induced pluripotent stem cells (iPSCs). Factors such as cytokines, extra cellular matrix components, and small molecules as well as the temporal association and concentration of these factors were tested on seven different human ESC and iPSC lines. We report the differentiation of up to 84% human CD45+ cells (average 41% ± 16%, from seven pluripotent lines) from the differentiation culture, including significant numbers of primitive CD45+/CD34+ and CD45+/CD34+/CD38- hematopoietic progenitors. Moreover, the numbers of hematopoietic progenitor cells generated, as measured by colony forming unit assays, were comparable to numbers obtained from fresh umbilical cord blood mononuclear cell isolates on a per CD45+ cell basis. Our approach demonstrates highly efficient generation of multipotent hematopoietic progenitors with among the highest efficiencies reported to date (CD45+/CD34+) using a single standardized differentiation protocol on several human ESC and iPSC lines. Our data add to the cumulating evidence for the existence of an in vitro derived precursor to the hematopoietic stem cell (HSC) with limited engrafting ability in transplanted mice but with multipotent hematopoietic potential. Because this protocol efficiently expands the preblood precursors and hematopoietic progenitors, it is ideal for testing novel factors for the generation and expansion of definitive HSCs with long-term repopulating ability.
Blood 2009 MAY
Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation.
Abstract
Abstract
Adult hematopoietic stem cells (HSCs) are routinely used to reconstitute hematopoiesis after myeloablation; however, transplantation efficacy and multilineage reconstitution can be limited by inadequate HSC number, or poor homing, engraftment, or self-renewal. Here we report that mouse and human HSCs express prostaglandin E2 (PGE2) receptors, and that short-term ex vivo exposure of HSCs to PGE2 enhances their homing, survival, and proliferation, resulting in increased long-term repopulating cell (LTRC) and competitive repopulating unit (CRU) frequency. HSCs pulsed with PGE2 are more competitive, as determined by head-to-head comparison in a competitive transplantation model. Enhanced HSC frequency and competitive advantage is stable and maintained upon serial transplantation, with full multilineage reconstitution. PGE2 increases HSC CXCR4 mRNA and surface expression, enhances their migration to SDF-1 in vitro and homing to bone marrow in vivo, and stimulates HSC entry into and progression through cell cycle. In addition, PGE2 enhances HSC survival, associated with an increase in Survivin mRNA and protein expression and reduction in intracellular active caspase-3. Our results define novel mechanisms of action whereby PGE2 enhances HSC function and supports a strategy to use PGE2 to facilitate hematopoietic transplantation.
The Journal of experimental medicine 2009 MAR
Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling.
Abstract
Abstract
Prostaglandins, particularly prostaglandin E2 (PGE2), play an important role during inflammation. This is exemplified by the clinical use of cyclooxygenase 2 inhibitors, which interfere with PGE2 synthesis, as effective antiinflammatory drugs. Here, we show that PGE2 directly promotes differentiation and proinflammatory functions of human and murine IL-17-producing T helper (Th17) cells. In human purified naive T cells, PGE2 acts via prostaglandin receptor EP2- and EP4-mediated signaling and cyclic AMP pathways to up-regulate IL-23 and IL-1 receptor expression. Furthermore, PGE2 synergizes with IL-1beta and IL-23 to drive retinoic acid receptor-related orphan receptor (ROR)-gammat, IL-17, IL-17F, CCL20, and CCR6 expression, which is consistent with the reported Th17 phenotype. While enhancing Th17 cytokine expression mainly through EP2, PGE2 differentially regulates interferon (IFN)-gamma production and inhibits production of the antiinflammatory cytokine IL-10 in Th17 cells predominantly through EP4. Furthermore, PGE2 is required for IL-17 production in the presence of antigen-presenting cells. Hence, the combination of inflammatory cytokines and noncytokine immunomodulators, such as PGE2, during differentiation and activation determines the ultimate phenotype of Th17 cells. These findings, together with the altered IL-12/IL-23 balance induced by PGE2 in dendritic cells, further highlight the crucial role of the inflammatory microenvironment in Th17 cell development and regulation.

 网站首页
网站首页