概要
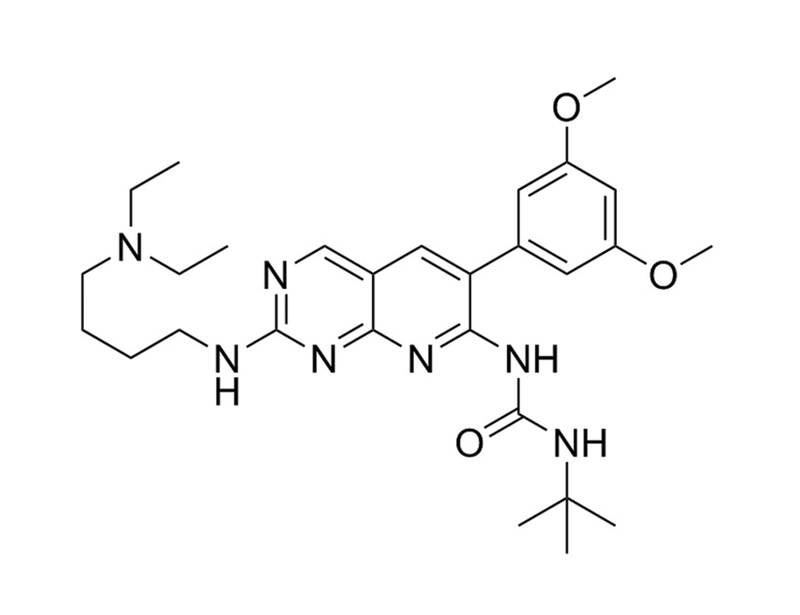
PD173074 is a selective and potent, ATP-competitive inhibitor of FGFR. It acts on both FGFR3 and FGFR1 (IC₅₀ = 5 and 21.5 nM respectively), and also inhibits FGFR2, FGFR4 and KDR. It is approximately 1000 times more potent than another common FGFR inhibitor SU5402. (Koziczak et al., Mohammadi et al., Trudel et al.)
REPROGRAMMING
· Prevents excision-mediated differentiation of mouse induced pluripotent stem cells generated using piggyBac transposons (Kaji et al.).
· Promotes reprogramming of human embryonic stem (ES) cells to naïve cells, or their maintenance in a naïve state, in combination with with Oct4, Klf4, and Klf2, LIF, CHIR99021 , and PD0325901. (Hanna et al.)
MAINTENANCE AND SELF-RENEWAL
· Suppresses the differentiation of mouse ES cells and maintains the undifferentiated state (Kunath et al., Ying et al.).
DIFFERENTIATION
· Blocks neural differentiation of mouse ES cells (Stavridis et al.).
· Promotes differentiation of human ES cells, but not when they are in a naïve or "ground" state (Hanna et al.).
REPROGRAMMING
· Prevents excision-mediated differentiation of mouse induced pluripotent stem cells generated using piggyBac transposons (Kaji et al.).
· Promotes reprogramming of human embryonic stem (ES) cells to naïve cells, or their maintenance in a naïve state, in combination with with Oct4, Klf4, and Klf2, LIF, CHIR99021 , and PD0325901. (Hanna et al.)
MAINTENANCE AND SELF-RENEWAL
· Suppresses the differentiation of mouse ES cells and maintains the undifferentiated state (Kunath et al., Ying et al.).
DIFFERENTIATION
· Blocks neural differentiation of mouse ES cells (Stavridis et al.).
· Promotes differentiation of human ES cells, but not when they are in a naïve or "ground" state (Hanna et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | PD173074 | 72164 | All | English |
| Safety Data Sheet | PD173074 | 72164 | All | English |
数据及文献
Publications (8)
Proceedings of the National Academy of Sciences of the United States of America 2010 MAY
Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs.
Abstract
Abstract
Human and mouse embryonic stem cells (ESCs) are derived from blastocyst-stage embryos but have very different biological properties, and molecular analyses suggest that the pluripotent state of human ESCs isolated so far corresponds to that of mouse-derived epiblast stem cells (EpiSCs). Here we rewire the identity of conventional human ESCs into a more immature state that extensively shares defining features with pluripotent mouse ESCs. This was achieved by ectopic induction of Oct4, Klf4, and Klf2 factors combined with LIF and inhibitors of glycogen synthase kinase 3beta (GSK3beta) and mitogen-activated protein kinase (ERK1/2) pathway. Forskolin, a protein kinase A pathway agonist which can induce Klf4 and Klf2 expression, transiently substitutes for the requirement for ectopic transgene expression. In contrast to conventional human ESCs, these epigenetically converted cells have growth properties, an X-chromosome activation state (XaXa), a gene expression profile, and a signaling pathway dependence that are highly similar to those of mouse ESCs. Finally, the same growth conditions allow the derivation of human induced pluripotent stem (iPS) cells with similar properties as mouse iPS cells. The generation of validated naïve" human ESCs will allow the molecular dissection of a previously undefined pluripotent state in humans and may open up new opportunities for patient-specific�
Nature 2009
Virus-free induction of pluripotency and subsequent excision of reprogramming factors
Abstract
Abstract
Reprogramming of somatic cells to pluripotency, thereby creating induced pluripotent stem (iPS) cells, promises to transform regenerative medicine. Most instances of direct reprogramming have been achieved by forced expression of defined factors using multiple viral vectors. However, such iPS cells contain a large number of viral vector integrations, any one of which could cause unpredictable genetic dysfunction. Whereas c-Myc is dispensable for reprogramming, complete elimination of the other exogenous factors is also desired because ectopic expression of either Oct4 (also known as Pou5f1) or Klf4 can induce dysplasia. Two transient transfection-reprogramming methods have been published to address this issue. However, the efficiency of both approaches is extremely low, and neither has been applied successfully to human cells so far. Here we show that non-viral transfection of a single multiprotein expression vector, which comprises the coding sequences of c-Myc, Klf4, Oct4 and Sox2 linked with 2A peptides, can reprogram both mouse and human fibroblasts. Moreover, the transgene can be removed once reprogramming has been achieved. iPS cells produced with this non-viral vector show robust expression of pluripotency markers, indicating a reprogrammed state confirmed functionally by in vitro differentiation assays and formation of adult chimaeric mice. When the single-vector reprogramming system was combined with a piggyBac transposon, we succeeded in establishing reprogrammed human cell lines from embryonic fibroblasts with robust expression of pluripotency markers. This system minimizes genome modification in iPS cells and enables complete elimination of exogenous reprogramming factors, efficiently providing iPS cells that are applicable to regenerative medicine, drug screening and the establishment of disease models.
Nature 2008 MAY
The ground state of embryonic stem cell self-renewal.
Abstract
Abstract
In the three decades since pluripotent mouse embryonic stem (ES) cells were first described they have been derived and maintained by using various empirical combinations of feeder cells, conditioned media, cytokines, growth factors, hormones, fetal calf serum, and serum extracts. Consequently ES-cell self-renewal is generally considered to be dependent on multifactorial stimulation of dedicated transcriptional circuitries, pre-eminent among which is the activation of STAT3 by cytokines (ref. 8). Here we show, however, that extrinsic stimuli are dispensable for the derivation, propagation and pluripotency of ES cells. Self-renewal is enabled by the elimination of differentiation-inducing signalling from mitogen-activated protein kinase. Additional inhibition of glycogen synthase kinase 3 consolidates biosynthetic capacity and suppresses residual differentiation. Complete bypass of cytokine signalling is confirmed by isolating ES cells genetically devoid of STAT3. These findings reveal that ES cells have an innate programme for self-replication that does not require extrinsic instruction. This property may account for their latent tumorigenicity. The delineation of minimal requirements for self-renewal now provides a defined platform for the precise description and dissection of the pluripotent state.
Development (Cambridge, England) 2007 AUG
A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification.
Abstract
Abstract
Neural tissue formation is induced by growth factors that activate networks of signal transduction cascades that ultimately lead to the expression of early neural genes, including transcription factors of the SoxB family. Here, we report that fibroblast growth factor (FGF)-induced Erk1/2 (Mapk3 and Mapk1, respectively) mitogen-activated protein kinase (MAPK), but not phosphatidylinositol 3'-OH kinase (PI3K, Pik3r1), signalling is required for neural specification in mouse embryonic stem (ES) cells and in the chick embryo. Further, blocking Erk1/2 inhibits the onset of key SoxB genes in both mouse ES cells (Sox1) and chick embryos (Sox2 and Sox3) and, in both contexts, Erk1/2 signalling is required during only a narrow time window, as neural specification takes place. In the absence of Erk1/2 signalling, differentiation of ES cells stalls following Fgf5 upregulation. Using differentiating ES cells as a model for neural specification, we demonstrate that sustained Erk1/2 activation controls the transition from an Fgf5-positive, primitive ectoderm-like cell state to a neural progenitor cell state without attenuating bone morphogenetic protein (BMP) signalling and we also define the minimum period of Erk1/2 activity required to mediate this key developmental step. Together, these findings identify a conserved, specific and stage-dependent requirement for Erk1/2 signalling downstream of FGF-induced neural specification in higher vertebrates and provide insight into the signalling dynamics governing this process.
Development (Cambridge, England) 2007 AUG
FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment.
Abstract
Abstract
Pluripotent embryonic stem (ES) cells must select between alternative fates of self-replication and lineage commitment during continuous proliferation. Here, we delineate the role of autocrine production of fibroblast growth factor 4 (Fgf4) and associated activation of the Erk1/2 (Mapk3/1) signalling cascade. Fgf4 is the major stimulus activating Erk in mouse ES cells. Interference with FGF or Erk activity using chemical inhibitors or genetic ablations does not impede propagation of undifferentiated ES cells. Instead, such manipulations restrict the ability of ES cells to commit to differentiation. ES cells lacking Fgf4 or treated with FGF receptor inhibitors resist neural and mesodermal induction, and are refractory to BMP-induced non-neural differentiation. Lineage commitment potential of Fgf4-null cells is restored by provision of FGF protein. Thus, FGF enables rather than antagonises the differentiation activity of BMP. The key downstream role of Erk signalling is revealed by examination of Erk2-null ES cells, which fail to undergo either neural or mesodermal differentiation in adherent culture, and retain expression of pluripotency markers Oct4, Nanog and Rex1. These findings establish that Fgf4 stimulation of Erk1/2 is an autoinductive stimulus for naïve ES cells to exit the self-renewal programme. We propose that the Erk cascade directs transition to a state that is responsive to inductive cues for germ layer segregation. Consideration of Erk signalling as a primary trigger that potentiates lineage commitment provides a context for reconciling disparate views on the contribution of FGF and BMP pathways during germ layer specification in vertebrate embryos.
Blood 2004 MAY
Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4;14) myeloma.
Abstract
Abstract
We have previously shown that dysregulation of fibroblast growth factor receptor 3 (FGFR3) by the t(4;14) translocation is a primary event in multiple myeloma (MM) and that activating mutations of FGFR3 are acquired in some cases. We describe here inhibition of wild-type (WT) and constitutively activated mutant FGFR3 autophosphorylation by the small molecule inhibitor, PD173074. Inhibition of FGFR3 in human myeloma cell lines was associated with decreased viability and tumor cell growth arrest. Further, morphologic, phenotypic, and functional changes typical of plasma cell (PC) differentiation, including increase in light-chain secretion and expression of CD31, were observed and this was followed by apoptosis. Finally, using a mouse model of FGFR3 myeloma, we demonstrate a delay in tumor progression and prolonged survival of mice treated with PD173074. These results indicate that inhibition of FGFR3, even in advanced disease associated with multiple genetic changes, may allow the cell to complete its developmental program and render it sensitive to apoptotic signals. In addition, this represents the validation of a therapeutic target in MM that may benefit patients who have a very poor prognosis with currently available treatments.

 网站首页
网站首页