概要
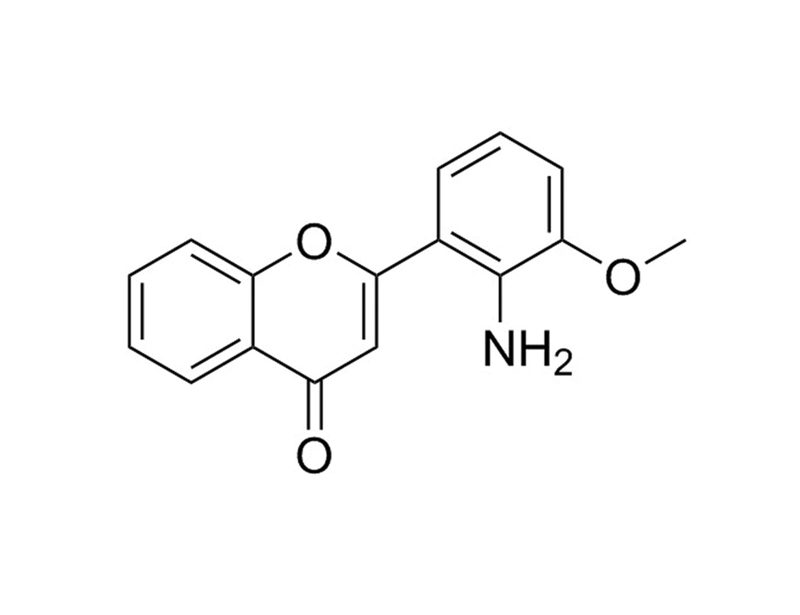
PD98059 is a selective, cell permeable inhibitor of the MEK/ERK pathway that acts by preventing the activation of MEK1 (IC₅₀ = 2 - 7 µM) and MEK2 (IC₅₀ = 50 µM) by upstream kinases. It does not inhibit activated MEK, or the p38 MAPK pathway. (Alessi et al., Davies et al., Dudley et al.)
MAINTENANCE AND SELF-RENEWAL
· Enhances the growth and self-renewal of mouse embryonic stem (ES) cells (Burdon et al., Qi et al.).
· Permits derivation of mouse ES cells from the refractory CBA mouse strain (Buehr and Smith).
DIFFERENTIATION
· Blocks the differentiation of mouse ES cells (Burdon et al.).
· Enhances adipogenic differentiation and blocks osteogenic differentiation of human mesenchymal stem cells (Jaiswal et al.).
CANCER RESEARCH
· Decreases number of AML blast colonies with minimal effect on normal hematopoietic progenitors (Milella et al.).
MAINTENANCE AND SELF-RENEWAL
· Enhances the growth and self-renewal of mouse embryonic stem (ES) cells (Burdon et al., Qi et al.).
· Permits derivation of mouse ES cells from the refractory CBA mouse strain (Buehr and Smith).
DIFFERENTIATION
· Blocks the differentiation of mouse ES cells (Burdon et al.).
· Enhances adipogenic differentiation and blocks osteogenic differentiation of human mesenchymal stem cells (Jaiswal et al.).
CANCER RESEARCH
· Decreases number of AML blast colonies with minimal effect on normal hematopoietic progenitors (Milella et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | PD98059 | 72172, 72174 | All | English |
| Safety Data Sheet | PD98059 | 72172, 72174 | All | English |
数据及文献
Publications (8)
Proceedings of the National Academy of Sciences of the United States of America 2004 APR
BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways.
Abstract
Abstract
The fate of pluripotent stem cells is tightly controlled during early embryonic development. Both the derivation and the maintenance of embryonic stem cells (ES cells) in vitro depend on feeder cell-derived growth factors that are largely unidentified. To dissect the mechanisms governing pluripotency, we conducted a screen to identify factors that are produced by mouse embryonic fibroblast STO cells and are required to maintain the pluripotency of ES cells. One of the factors is bone morphogenetic protein 4 (BMP4). Unexpectedly, the major effect of BMP4 on the self-renewal of ES cells is accomplished by means of the inhibition of both extracellular receptor kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) pathways, and inhibitors of ERK and p38 MAPKs mimic the effect of BMP4 on ES cells. Importantly, inhibition of the p38 MAPK pathway by SB203580 overcomes the block in deriving ES cells from blastocysts lacking a functional Alk3, the BMP type IA receptor. These results uncover a paradigm for BMP signaling in the biology of pluripotent stem cells.
Philosophical transactions of the Royal Society of London. Series B, Biological sciences 2003 AUG
Genesis of embryonic stem cells.
Abstract
Abstract
Embryonic stem (ES) cells are permanent pluripotent stem cell lines established from pre-implantation mouse embryos. There is currently great interest in the potential therapeutic applications of analogous cells derived from human embryos. The isolation of ES cells is commonly presented as a straightforward transfer of cells in the early embryo into culture. In reality, however, continuous expansion of pluripotent cells does not occur in vivo, and in vitro is the exception rather than the norm. Both genetic and epigenetic factors influence the ability to derive ES cells. We have tracked the expression of a key marker and determinant of pluripotency, the transcription factor Oct-4, in primary cultures of mouse epiblasts and used this to assay the effect of experimental manipulations on the maintenance of a pluripotent cell compartment. We find that expression of Oct-4 is often lost prior to overt cytodifferentiation of the epiblast. The rate and extent of Oct-4 extinction varies with genetic background. We report that treatment with the MAP kinase/ERK kinase inhibitor PD98059, which suppresses activation of the mitogen-activated protein kinases Erk1 and Erk2, results in increased persistence of Oct-4-expressing cells. Oct-4 expression is also relatively sustained in cultures of diapause embryos and of isolated inner cell masses. Combination of all three conditions allowed the derivation of germline-competent ES cells from the normally refractory CBA mouse strain. These findings suggest that the genesis of an ES cell is a relatively complex process requiring epigenetic modulation of key gene expression over a brief time-window. Procedures that extend this time-window and/or directly regulate the critical genes should increase the efficiency of ES cell derivation.
The Journal of clinical investigation 2001 SEP
Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia.
Abstract
Abstract
The mitogen-activated protein kinase (MAPK) pathway regulates growth and survival of many cell types, and its constitutive activation has been implicated in the pathogenesis of a variety of malignancies. In this study we demonstrate that small-molecule MEK inhibitors (PD98059 and PD184352) profoundly impair cell growth and survival of acute myeloid leukemia (AML) cell lines and primary samples with constitutive MAPK activation. These agents abrogate the clonogenicity of leukemic cells but have minimal effects on normal hematopoietic progenitors. MEK blockade also results in sensitization to spontaneous and drug-induced apoptosis. At a molecular level, these effects correlate with modulation of the expression of cyclin-dependent kinase inhibitors (p27(Kip1) and p21(Waf1/CIP1)) and antiapoptotic proteins of the inhibitor of apoptosis proteins (IAP) and Bcl-2 families. Interruption of constitutive MEK/MAPK signaling therefore represents a promising therapeutic strategy in AML.
The Biochemical journal 2000 OCT
Specificity and mechanism of action of some commonly used protein kinase inhibitors.
Abstract
Abstract
The specificities of 28 commercially available compounds reported to be relatively selective inhibitors of particular serine/threonine-specific protein kinases have been examined against a large panel of protein kinases. The compounds KT 5720, Rottlerin and quercetin were found to inhibit many protein kinases, sometimes much more potently than their presumed targets, and conclusions drawn from their use in cell-based experiments are likely to be erroneous. Ro 318220 and related bisindoylmaleimides, as well as H89, HA1077 and Y 27632, were more selective inhibitors, but still inhibited two or more protein kinases with similar potency. LY 294002 was found to inhibit casein kinase-2 with similar potency to phosphoinositide (phosphatidylinositol) 3-kinase. The compounds with the most impressive selectivity profiles were KN62, PD 98059, U0126, PD 184352, rapamycin, wortmannin, SB 203580 and SB 202190. U0126 and PD 184352, like PD 98059, were found to block the mitogen-activated protein kinase (MAPK) cascade in cell-based assays by preventing the activation of MAPK kinase (MKK1), and not by inhibiting MKK1 activity directly. Apart from rapamycin and PD 184352, even the most selective inhibitors affected at least one additional protein kinase. Our results demonstrate that the specificities of protein kinase inhibitors cannot be assessed simply by studying their effect on kinases that are closely related in primary structure. We propose guidelines for the use of protein kinase inhibitors in cell-based assays.
The Journal of biological chemistry 2000 MAR
Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase.
Abstract
Abstract
Adult human mesenchymal stem cells are primary, multipotent cells capable of differentiating to osteocytic, chondrocytic, and adipocytic lineages when stimulated under appropriate conditions. To characterize the molecular mechanisms that regulate osteogenic differentiation, we examined the contribution of mitogen-activated protein kinase family members, ERK, JNK, and p38. Treatment of these stem cells with osteogenic supplements resulted in a sustained phase of ERK activation from day 7 to day 11 that coincided with differentiation, before decreasing to basal levels. Activation of JNK occurred much later (day 13 to day 17) in the osteogenic differentiation process. This JNK activation was associated with extracellular matrix synthesis and increased calcium deposition, the two hallmarks of bone formation. Inhibition of ERK activation by PD98059, a specific inhibitor of the ERK signaling pathway, blocked the osteogenic differentiation in a dose-dependent manner, as did transfection with a dominant negative form of MAP kinase kinase (MEK-1). Significantly, the blockage of osteogenic differentiation resulted in the adipogenic differentiation of the stem cells and the expression of adipose-specific mRNAs peroxisome proliferator-activated receptor gamma2, aP2, and lipoprotein lipase. These observations provide a potential mechanism involving MAP kinase activation in osteogenic differentiation of adult stem cells and suggest that commitment of hMSCs into osteogenic or adipogenic lineages is governed by activation or inhibition of ERK, respectively.
Developmental biology 1999 JUN
Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells.
Abstract
Abstract
The propagation of pluripotent mouse embryonic stem (ES) cells depends on signals transduced through the cytokine receptor subunit gp130. Signalling molecules activated downstream of gp130 in ES cells include STAT3, the protein tyrosine phosphatase SHP-2, and the mitogen-activated protein kinases, ERK1 and ERK2. A chimaeric receptor in which tyrosine 118 in the gp130 cytoplasmic domain was mutated did not engage SHP-2 and failed to activate ERKs. However, this receptor did support ES cell self-renewal. In fact, stem cell colonies formed at 100-fold lower concentrations of cytokine than the unmodified receptor. Moreover, altered ES cell morphology and growth were observed at high cytokine concentrations. These indications of deregulated signalling in the absence of tyrosine 118 were substantiated by sustained activation of STAT3. Confirmation that ERK activation is not required for self-renewal was obtained by propagation of pluripotent ES cells in the presence of the MEK inhibitor PD098059. In fact, the growth of undifferentiated ES cells was enhanced by culture in PD098059. Thus activation of ERKs appears actively to impair self-renewal. These data imply that the self-renewal signal from gp130 is a finely tuned balance of positive and negative effectors.

 网站首页
网站首页