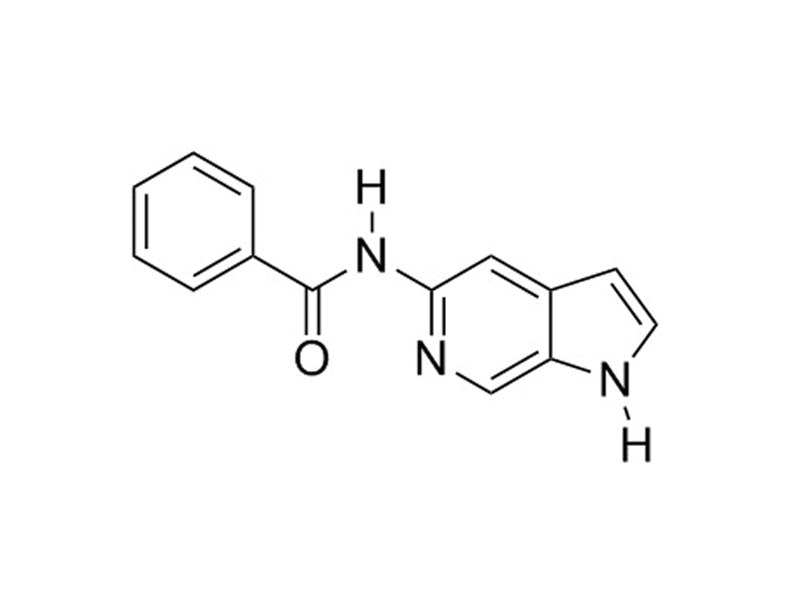
概要
OAC1 is an OCT-4-activating compound that activates expression through the OCT-4 gene promoter (Li et al.). Oct-4 (POU5F1) is a transcription factor that is critically involved in the self-renewal of pluripotent stem cells, and its expression is commonly used as a marker for pluripotency.
REPROGRAMMING
· Enhances the reprogramming efficiency of mouse embryonic fibroblasts transfected with OCT4, SOX2, KLF4, and c-MYC (Li et al.).
MAINTENANCE AND SELF-RENEWAL
· Mediates ex vivo expansion of cord blood CD34+ hematopoietic stem and progenitor cells (Huang et al.).
REPROGRAMMING
· Enhances the reprogramming efficiency of mouse embryonic fibroblasts transfected with OCT4, SOX2, KLF4, and c-MYC (Li et al.).
MAINTENANCE AND SELF-RENEWAL
· Mediates ex vivo expansion of cord blood CD34+ hematopoietic stem and progenitor cells (Huang et al.).
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | OAC1 | 72602 | All | English |
| Safety Data Sheet | OAC1 | 72602 | All | English |
数据及文献
Publications (4)
Leukemia 2016 JAN
Activation of OCT4 enhances ex vivo expansion of human cord blood hematopoietic stem and progenitor cells by regulating HOXB4 expression.
Abstract
Abstract
Although hematopoietic stem cells (HSC) are the best characterized and the most clinically used adult stem cells, efforts are still needed to understand how to best ex vivo expand these cells. Here we present our unexpected finding that OCT4 is involved in the enhancement of cytokine-induced expansion capabilities of human cord blood (CB) HSC. Activation of OCT4 by Oct4-activating compound 1 (OAC1) in CB CD34(+) cells enhanced ex vivo expansion of HSC, as determined by a rigorously defined set of markers for human HSC, and in vivo short-term and long-term repopulating ability in NSG mice. Limiting dilution analysis revealed that OAC1 treatment resulted in 3.5-fold increase in the number of SCID repopulating cells (SRCs) compared with that in day 0 uncultured CD34(+) cells and 6.3-fold increase compared with that in cells treated with control vehicle. Hematopoietic progenitor cells, as assessed by in vitro colony formation, were also enhanced. Furthermore, we showed that OAC1 treatment led to OCT4-mediated upregulation of HOXB4. Consistently, siRNA-mediated knockdown of HOXB4 expression suppressed effects of OAC1 on ex vivo expansion of HSC. Our study has identified the OCT4-HOXB4 axis in ex vivo expansion of human CB HSC.
Proceedings of the National Academy of Sciences of the United States of America 2012 DEC
Identification of Oct4-activating compounds that enhance reprogramming efficiency.
Abstract
Abstract
One of the hurdles for practical application of induced pluripotent stem cells (iPSC) is the low efficiency and slow process of reprogramming. Octamer-binding transcription factor 4 (Oct4) has been shown to be an essential regulator of embryonic stem cell (ESC) pluripotency and key to the reprogramming process. To identify small molecules that enhance reprogramming efficiency, we performed a cell-based high-throughput screening of chemical libraries. One of the compounds, termed Oct4-activating compound 1 (OAC1), was found to activate both Oct4 and Nanog promoter-driven luciferase reporter genes. Furthermore, when added to the reprogramming mixture along with the quartet reprogramming factors (Oct4, Sox2, c-Myc, and Klf4), OAC1 enhanced the iPSC reprogramming efficiency and accelerated the reprogramming process. Two structural analogs of OAC1 also activated Oct4 and Nanog promoters and enhanced iPSC formation. The iPSC colonies derived using the Oct4-activating compounds along with the quartet factors exhibited typical ESC morphology, gene-expression pattern, and developmental potential. OAC1 seems to enhance reprogramming efficiency in a unique manner, independent of either inhibition of the p53-p21 pathway or activation of the Wnt-β-catenin signaling. OAC1 increases transcription of the Oct4-Nanog-Sox2 triad and Tet1, a gene known to be involved in DNA demethylation.
Cell 2007 NOV
Induction of pluripotent stem cells from adult human fibroblasts by defined factors.
Abstract
Abstract
Successful reprogramming of differentiated human somatic cells into a pluripotent state would allow creation of patient- and disease-specific stem cells. We previously reported generation of induced pluripotent stem (iPS) cells, capable of germline transmission, from mouse somatic cells by transduction of four defined transcription factors. Here, we demonstrate the generation of iPS cells from adult human dermal fibroblasts with the same four factors: Oct3/4, Sox2, Klf4, and c-Myc. Human iPS cells were similar to human embryonic stem (ES) cells in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity. Furthermore, these cells could differentiate into cell types of the three germ layers in vitro and in teratomas. These findings demonstrate that iPS cells can be generated from adult human fibroblasts.
Nature genetics 2000 APR
Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells.
Abstract
Abstract
Cell fate during development is defined by transcription factors that act as molecular switches to activate or repress specific gene expression programmes. The POU transcription factor Oct-3/4 (encoded by Pou5f1) is a candidate regulator in pluripotent and germline cells and is essential for the initial formation of a pluripotent founder cell population in the mammalian embryo. Here we use conditional expression and repression in embryonic stem (ES) cells to determine requirements for Oct-3/4 in the maintenance of developmental potency. Although transcriptional determination has usually been considered as a binary on-off control system, we found that the precise level of Oct-3/4 governs three distinct fates of ES cells. A less than twofold increase in expression causes differentiation into primitive endoderm and mesoderm. In contrast, repression of Oct-3/4 induces loss of pluripotency and dedifferentiation to trophectoderm. Thus a critical amount of Oct-3/4 is required to sustain stem-cell self-renewal, and up- or downregulation induce divergent developmental programmes. Our findings establish a role for Oct-3/4 as a master regulator of pluripotency that controls lineage commitment and illustrate the sophistication of critical transcriptional regulators and the consequent importance of quantitative analyses.

 网站首页
网站首页