概要
Primary human mononuclear cells (MNCs) were isolated from peripheral blood (PB) leukapheresis samples using density gradient separation and/or red blood cell lysis. PB was collected using acid-citrate-dextrose solution A (ACDA) as the anticoagulant. High-resolution HLA typing for Class I and Class II alleles and CMV status are available upon request.
Cells were obtained using Institutional Review Board (IRB)-approved consent forms and protocols.
Certain products are only available in select territories. Please contact your local Sales representative or Product & Scientific Support at techsupport@stemcell.com for further information.
Browse our Frequently Asked Questions (FAQs) on Primary Cells.
Cells were obtained using Institutional Review Board (IRB)-approved consent forms and protocols.
Certain products are only available in select territories. Please contact your local Sales representative or Product & Scientific Support at techsupport@stemcell.com for further information.
Browse our Frequently Asked Questions (FAQs) on Primary Cells.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
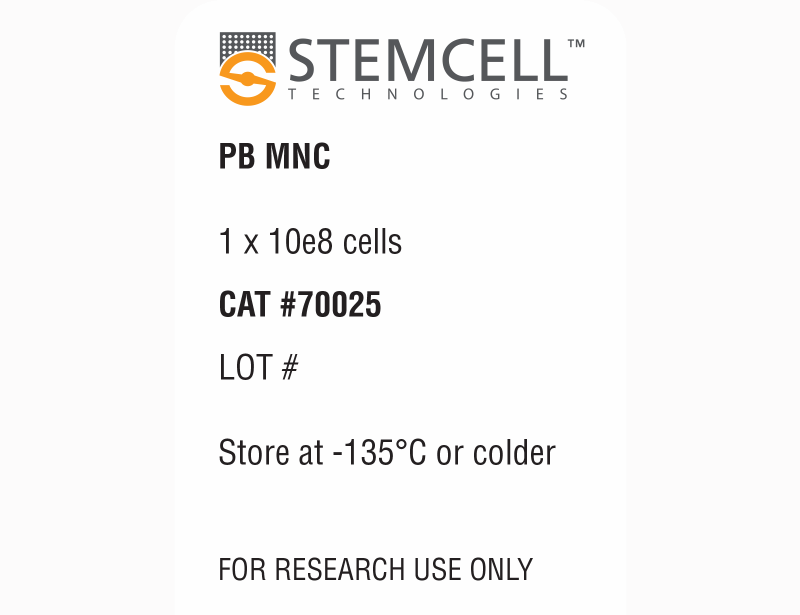
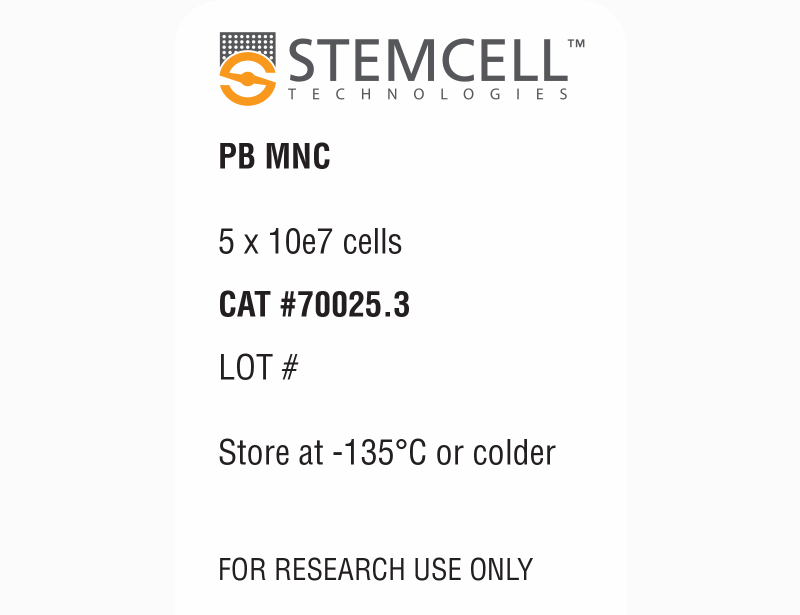
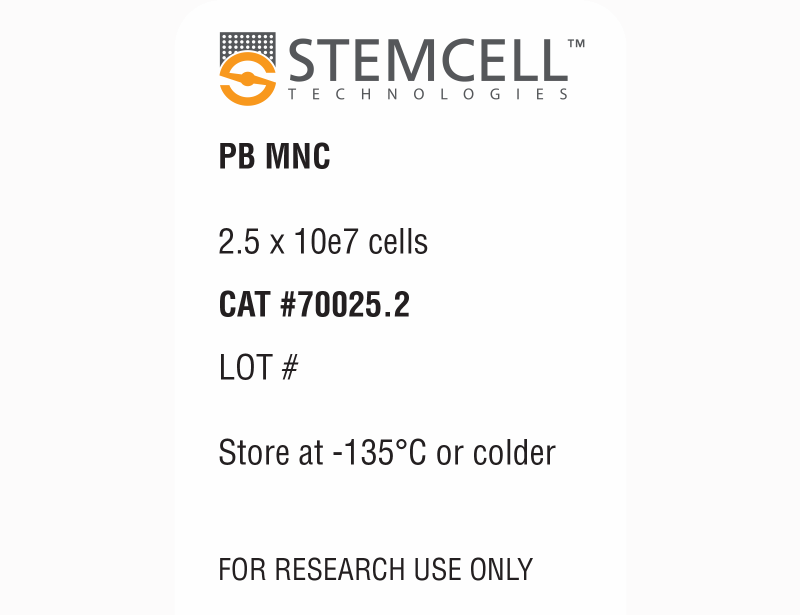
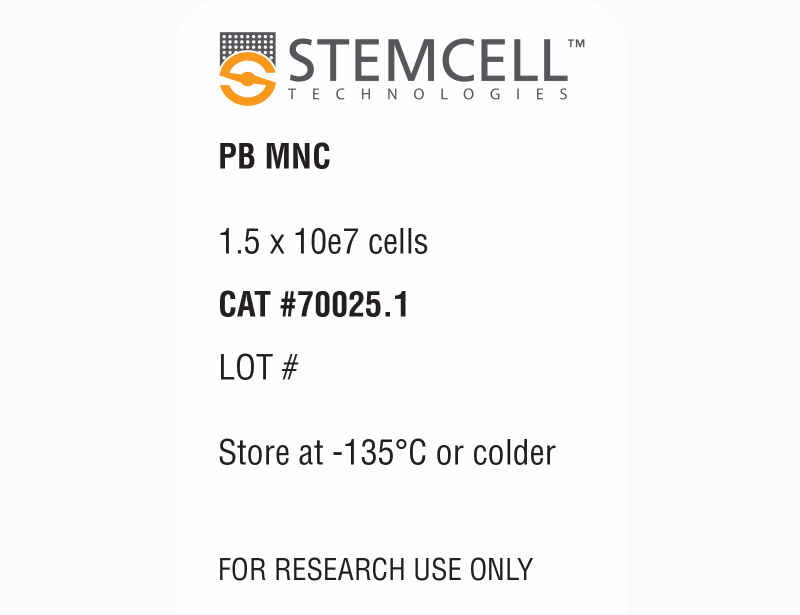
| Product Information Sheet | Human Peripheral Blood Mononuclear Cells, Frozen | 70025, 70025.1, 70025.2, 70025.3 | All | English |
数据及文献
Publications (12)
Cancer cell 2018 FEB
Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1.
Abstract
Abstract
Protein glycosylation provides proteomic diversity in regulating protein localization, stability, and activity; it remains largely unknown whether the sugar moiety contributes to immunosuppression. In the study of immune receptor glycosylation, we showed that EGF induces programmed death ligand 1 (PD-L1) and receptor programmed cell death protein 1 (PD-1) interaction, requiring beta$-1,3-N-acetylglucosaminyl transferase (B3GNT3) expression in triple-negative breast cancer. Downregulation of B3GNT3 enhances cytotoxic T cell-mediated anti-tumor immunity. A monoclonal antibody targeting glycosylated PD-L1 (gPD-L1) blocks PD-L1/PD-1 interaction and promotes PD-L1 internalization and degradation. In addition to immune reactivation, drug-conjugated gPD-L1 antibody induces a potent cell-killing effect as well as a bystander-killing effect on adjacent cancer cells lacking PD-L1 expression without any detectable toxicity. Our work suggests targeting protein glycosylation as a potential strategy to enhance immune checkpoint therapy.
Immunity 2017 AUG
Dendritic Cells but Not Macrophages Sense Tumor Mitochondrial DNA for Cross-priming through Signal Regulatory Protein α Signaling.
Abstract
Abstract
Inhibition of cytosolic DNA sensing represents a strategy that tumor cells use for immune evasion, but the underlying mechanisms are unclear. Here we have shown that CD47-signal regulatory protein α (SIRPα) axis dictates the fate of ingested DNA in DCs for immune evasion. Although macrophages were more potent in uptaking tumor DNA, increase of DNA sensing by blocking the interaction of SIRPα with CD47 preferentially occurred in dendritic cells (DCs) but not in macrophages. Mechanistically, CD47 blockade enabled the activation of NADPH oxidase NOX2 in DCs, which in turn inhibited phagosomal acidification and reduced the degradation of tumor mitochondrial DNA (mtDNA) in DCs. mtDNA was recognized by cyclic-GMP-AMP synthase (cGAS) in the DC cytosol, contributing to type I interferon (IFN) production and antitumor adaptive immunity. Thus, our findings have demonstrated how tumor cells inhibit innate sensing in DCs and suggested that the CD47-SIRPα axis is critical for DC-driven antitumor immunity.
Developmental cell 2017 APR
Phosphorylation of NEUROG3 Links Endocrine Differentiation to the Cell Cycle in Pancreatic Progenitors.
Abstract
Abstract
During pancreatic development, proliferating pancreatic progenitors activate the proendocrine transcription factor neurogenin 3 (NEUROG3), exit the cell cycle, and differentiate into islet cells. The mechanisms that direct robust NEUROG3 expression within a subset of progenitor cells control the size of the endocrine population. Here we demonstrate that NEUROG3 is phosphorylated within the nucleus on serine 183, which catalyzes its hyperphosphorylation and proteosomal degradation. During progression through the progenitor cell cycle, NEUROG3 phosphorylation is driven by the actions of cyclin-dependent kinases 2 and 4/6 at G1/S cell-cycle checkpoint. Using models of mouse and human pancreas development, we show that lengthening of the G1 phase of the pancreatic progenitor cell cycle is essential for proper induction of NEUROG3 and initiation of endocrine cell differentiation. In sum, these studies demonstrate that progenitor cell-cycle G1 lengthening, through its actions on stabilization of NEUROG3, is an essential variable in normal endocrine cell genesis.
Journal of biotechnology 2016 MAY
A novel approach for emerging and antibiotic resistant infections: Innate defense regulators as an agnostic therapy.
Abstract
Abstract
Innate Defense Regulators (IDRs) are short synthetic peptides that target the host innate immune system via an intracellular adaptor protein which functions at key signaling nodes. In this work, further details of the mechanism of action of IDRs have been discovered. The studies reported here show that the lead clinical IDR, SGX94, has broad-spectrum activity against Gram-negative and Gram-positive bacterial infections caused by intracellular or extracellular bacteria and also complements the actions of standard of care antibiotics. Based on in vivo and primary cell culture studies, this activity is shown to result from the primary action of SGX94 on tissue-resident cells and subsequent secondary signaling to activate myeloid-derived cells, resulting in enhanced bacterial clearance and increased survival. Data from non-clinical and clinical studies also show that SGX94 treatment modulates pro-inflammatory and anti-inflammatory cytokine levels, thereby mitigating the deleterious inflammatory consequences of innate immune activation. Since they act through host pathways to provide both broad-spectrum anti-infective capability as well as control of inflammation, IDRs are unlikely to be impacted by resistance mechanisms and offer potential clinical advantages in the fight against emerging and antibiotic resistant bacterial infections.
Organic & biomolecular chemistry 2016 JUL
Cellular thermal shift and clickable chemical probe assays for the determination of drug-target engagement in live cells.
Abstract
Abstract
Proof of drug-target engagement in physiologically-relevant contexts is a key pillar of successful therapeutic target validation. We developed two orthogonal technologies, the cellular thermal shift assay (CETSA) and a covalent chemical probe reporter approach (harnessing sulfonyl fluoride tyrosine labeling and subsequent click chemistry) to measure the occupancy of the mRNA-decapping scavenger enzyme DcpS by a small molecule inhibitor in live cells. Enzyme affinity determined using isothermal dose response fingerprinting (ITDRFCETSA) and the concentration required to occupy 50% of the enzyme (OC50) using the chemical probe reporter assay were very similar. In this case, the chemical probe method worked well due to the long offset kinetics of the reversible inhibitor (determined using a fluorescent dye-tagged probe). This work suggests that CETSA could become the first choice assay to determine in-cell target engagement due to its simplicity.
Analytical chemistry 2016 APR
Water-in-Water Droplets by Passive Microfluidic Flow Focusing.
Abstract
Abstract
We present a simple microfluidic system that generates water-in-water, aqueous two phase system (ATPS) droplets, by passive flow focusing. ATPS droplet formation is achieved by applying weak hydrostatic pressures, with liquid-filled pipette tips as fluid columns at the inlets, to introduce low speed flows to the flow focusing junction. To control the size of the droplets, we systematically vary the interfacial tension and viscosity of the ATPS fluids and adjust the fluid column height at the fluid inlets. The size of the droplets scales with a power law of the ratio of viscous stresses in the two ATPS phases. Overall, we find a drop size coefficient of variation (CV; i.e., polydispersity) of about 10%. We also find that when drops form very close to the flow focusing junction, the drops have a CV of less than 1%. Our droplet generation method is easily scalable: we demonstrate a parallel system that generates droplets simultaneously and improves the droplet production rate by up to one order of magnitude. Finally, we show the potential application of our system for encapsulating cells in water-in-water emulsions by encapsulating microparticles and cells. To the best of our knowledge, our microfluidic technique is the first that forms low interfacial tension ATPS droplets without applying external perturbations. We anticipate that this simple approach will find utility in drug and cell delivery applications because of the all-biocompatible nature of the water-in-water ATPS environment.

 网站首页
网站首页