概要
The assay is based on the sandwich ELISA method, in which samples are added to ELISA strip plates pre-coated with capture antibodies specific for the cytokine. The captured cytokine is detected by addition of a biotinylated detection antibody, followed by streptavidin-horseradish peroxidase, which binds the biotinylated antibody. Addition of the chromogenic enzyme substrate 3,3’,5,5’ tetramethylbenzidine (TMB) results in a colored product with an intensity directly proportional to the concentration of cytokine in the sample. The concentration of the cytokine is determined by comparison to a serial dilution of the cytokine standard analyzed in parallel.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 1 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 2 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 3 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 4 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 5 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 6 | Human IFN-gamma ELISA Kit | 02002 | All | English |
| Safety Data Sheet 7 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 8 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 9 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 10 | Human IFN-gamma ELISA Kit | 02002, 02003 | All | English |
| Safety Data Sheet 1 | Human IFN-gamma ELISA Kit | 02003 | All | English |
数据及文献
Data
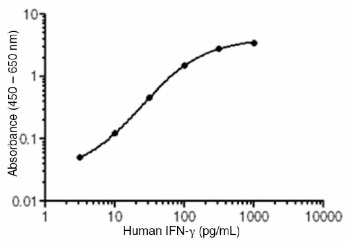
Representative Standard Curve
• Reportable range: 3.2 - 1000 pg/mL. This is the concentration range in which measurement of the analyte can be done with the highest precision, accuracy, and linearity.
• Sensitivity: The limit of detection of this assay is 1 pg/mL. This is the analyte concentration with absorbance two standard deviations higher than the zero standard.
• Accuracy: The analyte standard of this ELISA was calibrated against NIAID* international standard Gxg01-902-535.
• Recovery: A mid-curve recovery of 85 - 91% was determined by spiking defined amounts of analyte standard into serum or plasma samples in repeated experiments.
• Precision: The intra-assay precision of this assay is 3.9% (CV). The inter-assay precision of this assay is 4.4% (CV).
*National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892-6612, USA.

 网站首页
网站首页