概要
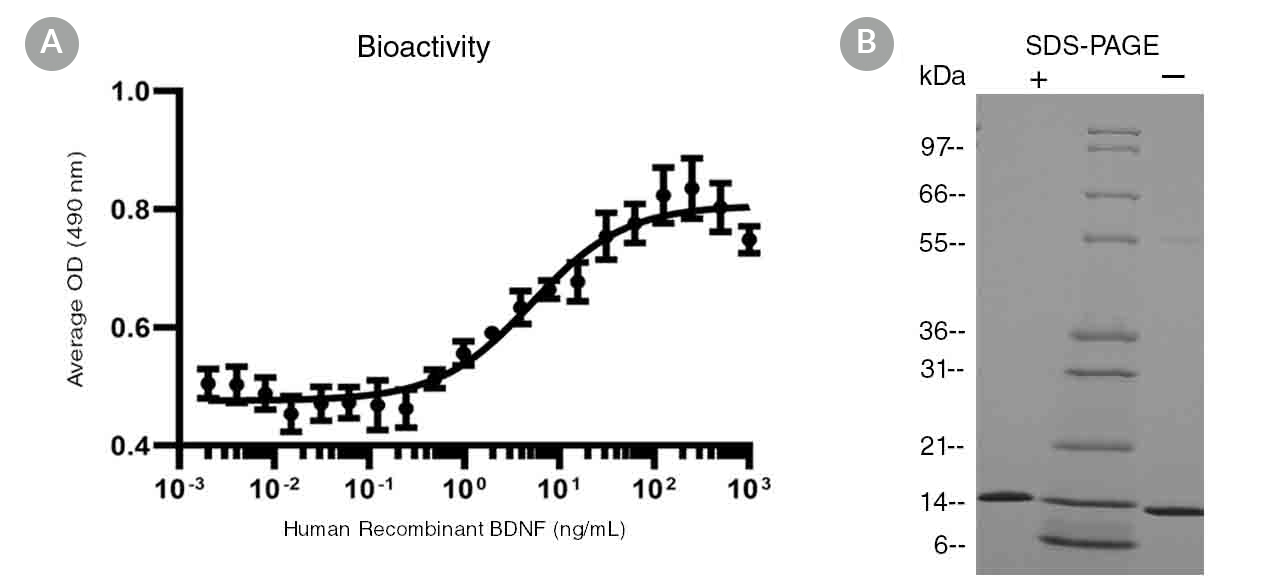
Brain-derived neurotrophic factor (BDNF), like nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), is a member of the NGF family of neurotrophins, which are required for the differentiation and survival of specific neuronal subpopulations in both the central and the peripheral nervous systems (Minichiello & Klein; Minichiello et al.). BDNF binds with high affinity to the tropomyosin receptor kinase B (TrkB), and activates AKT and ERK pathways (Mattson et al.). It is expressed in the hippocampus, cortex, and synapses of the basal forebrain. BDNF acts as a survival factor for human embryonic stem cells when plated on either feeder cells or Corning® Matrigel® (Pyle et al.). BDNF regulates synaptic transmission and plasticity at adult synapses in the central nervous system, and contributes to adaptive neuronal responses including long-term potentiation, long-term depression, certain forms of short-term synaptic plasticity, and homeostatic regulation of neuronal excitability (Reichardt). It also has a role in neurogenesis by promoting survival and growth of dorsal root ganglion cells, and hippocampal and cortical neurons (Binder & Scharfman). BDNF, together with glial cell line-derived neurotrophic factor (GDNF) and other supplements, is commonly used to differentiate human pluripotent stem cell (hPSC)-derived neural progenitor cells into neurons (Brafman).
数据及文献
Publications (1)
STEM CELLS Translational Medicine 2016 NOV
A Novel Protocol for Directed Differentiation of C9orf72-Associated Human Induced Pluripotent Stem Cells Into Contractile Skeletal Myotubes
Swartz EW et al.
Abstract
: Induced pluripotent stem cells (iPSCs) offer an unlimited resource of cells to be used for the study of underlying molecular biology of disease, therapeutic drug screening, and transplant-based regenerative medicine. However, methods for the directed differentiation of skeletal muscle for these purposes remain scarce and incomplete. Here, we present a novel, small molecule-based protocol for the generation of multinucleated skeletal myotubes using eight independent iPSC lines. Through combinatorial inhibition of phosphoinositide 3-kinase (PI3K) and glycogen synthase kinase 3β (GSK3β) with addition of bone morphogenic protein 4 (BMP4) and fibroblast growth factor 2 (FGF2), we report up to 64% conversion of iPSCs into the myogenic program by day 36 as indicated by MYOG+ cell populations. These cells began to exhibit spontaneous contractions as early as 34 days in vitro in the presence of a serum-free medium formulation. We used this protocol to obtain iPSC-derived muscle cells from frontotemporal dementia (FTD) patients harboring C9orf72 hexanucleotide repeat expansions (rGGGGCC), sporadic FTD, and unaffected controls. iPSCs derived from rGGGGCC carriers contained RNA foci but did not vary in differentiation efficiency when compared to unaffected controls nor display mislocalized TDP-43 after as many as 120 days in vitro. This study presents a rapid, efficient, and transgene-free method for generating multinucleated skeletal myotubes from iPSCs and a resource for further modeling the role of skeletal muscle in amyotrophic lateral sclerosis and other motor neuron diseases. SIGNIFICANCE Protocols to produce skeletal myotubes for disease modeling or therapy are scarce and incomplete. The present study efficiently generates functional skeletal myotubes from human induced pluripotent stem cells using a small molecule-based approach. Using this strategy, terminal myogenic induction of up to 64% in 36 days and spontaneously contractile myotubes within 34 days were achieved. Myotubes derived from patients carrying the C9orf72 repeat expansion show no change in differentiation efficiency and normal TDP-43 localization after as many as 120 days in vitro when compared to unaffected controls. This study provides an efficient, novel protocol for the generation of skeletal myotubes from human induced pluripotent stem cells that may serve as a valuable tool in drug discovery and modeling of musculoskeletal and neuromuscular diseases.
View All Publications

 网站首页
网站首页