概要
mFreSR™ is a serum-free cryopreservation medium designed for the cryopreservation of human embryonic and induced pluripotent stem cells (ES and iPS cells). mFreSR™ contains DMSO and is complete and ready-to-use. Together with mTeSR™1, TeSR™2, or mTeSR™ Plus, mFreSR™ eliminates the use of feeders and serum. Human ES and iPS cells cryopreserved in mFreSR™ have thawing efficiencies higher than reported conventional thawing methods using serum.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | mFreSR™ | 05854, 05855 | All | English |
| Safety Data Sheet | mFreSR™ | 05854, 05855 | All | English |
| Safety Data Sheet 1 | mFreSR™ | 05855 | All | English |
| Safety Data Sheet 2 | mFreSR™ | 05855 | All | English |
数据及文献
Data
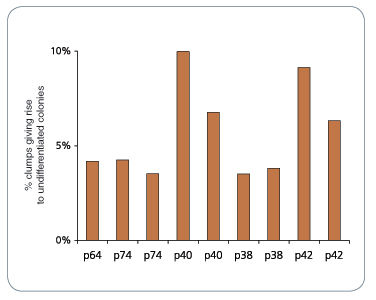
Figure 1. mFreSR™ Improves Thawing Efficiencies 5- to 10-Fold over Other Reported Methods
H9 hESCs were cryopreserved in mFreSR™ at the indicated passage number. Thawing efficiencies were analyzed by counting the number of surviving clumps after thawing.
Publications (14)
PloS one 2017
Functional Maturation of Human Stem Cell-Derived Neurons in Long-Term Cultures.
Abstract
Abstract
Differentiated neurons can be rapidly acquired, within days, by inducing stem cells to express neurogenic transcription factors. We developed a protocol to maintain long-term cultures of human neurons, called iNGNs, which are obtained by inducing Neurogenin-1 and Neurogenin-2 expression in induced pluripotent stem cells. We followed the functional development of iNGNs over months and they showed many hallmark properties for neuronal maturation, including robust electrical and synaptic activity. Using iNGNs expressing a variant of channelrhodopsin-2, called CatCh, we could control iNGN activity with blue light stimulation. In combination with optogenetic tools, iNGNs offer opportunities for studies that require precise spatial and temporal resolution. iNGNs developed spontaneous network activity, and these networks had excitatory glutamatergic synapses, which we characterized with single-cell synaptic recordings. AMPA glutamatergic receptor activity was especially dominant in postsynaptic recordings, whereas NMDA glutamatergic receptor activity was absent from postsynaptic recordings but present in extrasynaptic recordings. Our results on long-term cultures of iNGNs could help in future studies elucidating mechanisms of human synaptogenesis and neurotransmission, along with the ability to scale-up the size of the cultures.
Stem Cell Research 2016 MAR
Generation of spinocerebellar ataxia type 3 patient-derived induced pluripotent stem cell line SCA3.B11.
Abstract
Abstract
Spinocerebellar ataxia type 3 (SCA3) is a dominantly inherited neurodegenerative disease caused by an expansion of the CAG-repeat in ATXN3. In this study, induced pluripotent stem cells (iPSCs) were generated from SCA3 patient dermal fibroblasts by electroporation with episomal plasmids encoding L-MYC, LIN28, SOX2, KLF4, OCT4 and short hairpin RNA targeting P53. The resulting iPSCs had normal karyotype, were free of integrated episomal plasmids, expressed pluripotency markers, could differentiate into the three germ layers in vitro and retained the disease-causing ATXN3 mutation. Potentially, this iPSC line could be a useful tool for the investigation of SCA3 disease mechanisms.
Molecular Neurodegeneration 2016 DEC
Manifestation of Huntington's disease pathology in human induced pluripotent stem cell-derived neurons.
Abstract
Abstract
Background: Huntington's disease (HD) is an incurable hereditary neurodegenerative disorder, which manifests itself as a loss of GABAergic medium spiny (GABA MS) neurons in the striatum and caused by an expansion of the CAG repeat in exon 1 of the huntingtin gene. There is no cure for HD, existing pharmaceutical can only relieve its symptoms. Results: Here, induced pluripotent stem cells were established from patients with low CAG repeat expansion in the huntingtin gene, and were then efficiently differentiated into GABA MS-like neurons (GMSLNs) under defined culture conditions. The generated HD GMSLNs recapitulated disease pathology in vitro, as evidenced by mutant huntingtin protein aggregation, increased number of lysosomes/autophagosomes, nuclear indentations, and enhanced neuronal death during cell aging. Moreover, store-operated channel (SOC) currents were detected in the differentiated neurons, and enhanced calcium entry was reproducibly demonstrated in all HD GMSLNs genotypes. Additionally, the quinazoline derivative, EVP4593, reduced the number of lysosomes/autophagosomes and SOC currents in HD GMSLNs and exerted neuroprotective effects during cell aging. Conclusions: Our data is the first to demonstrate the direct link of nuclear morphology and SOC calcium deregulation to mutant huntingtin protein expression in iPSCs-derived neurons with disease-mimetic hallmarks, providing a valuable tool for identification of candidate anti-HD drugs. Our experiments demonstrated that EVP4593 may be a promising anti-HD drug. [ABSTRACT FROM AUTHOR]
2015
Microarray Approach to Identify the Signaling Network Responsible for Self-Renewal of Human Embryonic Stem Cells
Abstract
Abstract
Here we introduce the representative method to culture HESCs under the feeder and feeder-free conditions, the former of which is used to maintain or expand undifferentiated HESCs, and the latter can be used for the preparation of pure HESCs RNA samples, or for screening factors influential on self-renewal of HESCs. We also describe a protocol and tips for conducting gene chip analysis focusing on widely used Affymetrix Microarrays. These techniques will provide us unprecedented scale of biological information that would illuminate a key to decipher complex signaling networks controlling pluripotency.
Nature Medicine 2014 NOV
An in vivo model of human small intestine using pluripotent stem cells.
Abstract
Abstract
Differentiation of human pluripotent stem cells (hPSCs) into organ-specific subtypes offers an exciting avenue for the study of embryonic development and disease processes, for pharmacologic studies and as a potential resource for therapeutic transplant. To date, limited in vivo models exist for human intestine, all of which are dependent upon primary epithelial cultures or digested tissue from surgical biopsies that include mesenchymal cells transplanted on biodegradable scaffolds. Here, we generated human intestinal organoids (HIOs) produced in vitro from human embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) that can engraft in vivo. These HIOs form mature human intestinal epithelium with intestinal stem cells contributing to the crypt-villus architecture and a laminated human mesenchyme, both supported by mouse vasculature ingrowth. In vivo transplantation resulted in marked expansion and maturation of the epithelium and mesenchyme, as demonstrated by differentiated intestinal cell lineages (enterocytes, goblet cells, Paneth cells, tuft cells and enteroendocrine cells), presence of functional brush-border enzymes (lactase, sucrase-isomaltase and dipeptidyl peptidase 4) and visible subepithelial and smooth muscle layers when compared with HIOs in vitro. Transplanted intestinal tissues demonstrated digestive functions as shown by permeability and peptide uptake studies. Furthermore, transplanted HIO-derived tissue was responsive to systemic signals from the host mouse following ileocecal resection, suggesting a role for circulating factors in the intestinal adaptive response. This model of the human small intestine may pave the way for studies of intestinal physiology, disease and translational studies.
Molecular systems biology 2014 NOV
Rapid neurogenesis through transcriptional activation in human stem cells.
Abstract
Abstract
Advances in cellular reprogramming and stem cell differentiation now enable ex vivo studies of human neuronal differentiation. However, it remains challenging to elucidate the underlying regulatory programs because differentiation protocols are laborious and often result in low neuron yields. Here, we overexpressed two Neurogenin transcription factors in human-induced pluripotent stem cells and obtained neurons with bipolar morphology in 4 days, at greater than 90% purity. The high purity enabled mRNA and microRNA expression profiling during neurogenesis, thus revealing the genetic programs involved in the rapid transition from stem cell to neuron. The resulting cells exhibited transcriptional, morphological and functional signatures of differentiated neurons, with greatest transcriptional similarity to prenatal human brain samples. Our analysis revealed a network of key transcription factors and microRNAs that promoted loss of pluripotency and rapid neurogenesis via progenitor states. Perturbations of key transcription factors affected homogeneity and phenotypic properties of the resulting neurons, suggesting that a systems-level view of the molecular biology of differentiation may guide subsequent manipulation of human stem cells to rapidly obtain diverse neuronal types.

 网站首页
网站首页