概要
技术资料
| ocument Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | CryoStor® CS10 | 07930, 07931, 07940, 07955, 07959, 07952 | All | English |
| Special Protocol | CryoStor® CS10 | 07930 | All | English |
| Safety Data Sheet | CryoStor® CS10 | 07930, 07931, 07940, 07955, 07959 | All | English |
数据及文献
Data
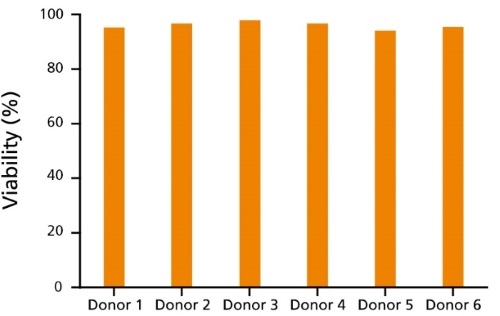
Figure 1. Immune Cells Cryopreserved in CryoStor®CS10 Show Reproducibly High Post-Thaw Cell Viability
CryoStor®CS10 effectively mitigates temperature-induced molecular cell stress responses to maximize post-thaw viability and recovery for a variety of immune cell types, including T cells (data not shown) and B cells. Here, human B cells from 6 different donors cryopreserved in CryoStor®CS10 show reproducibly high viability after thawing, as measured by Propidium Iodide staining (ranging from 94.3 - 97.9%).
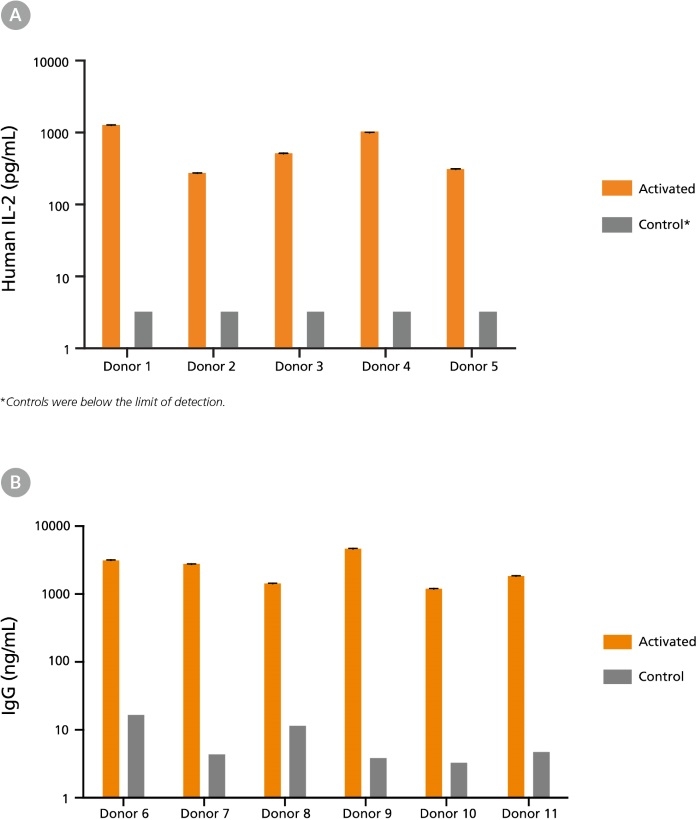
Figure 2. Immune Cells Cryopreserved in CryoStor®CS10 Retain Functionality Post-Thaw
(A) Human peripheral blood Pan-T cells cryopreserved in CryoStor®CS10 were thawed and cultured with or without the addition of T cell activating factors. Cells from Donors 1-3 were cultured in RPMI Medium supplemented with 10% FBS, with (activated) or without (control) 40 ng/mL PMA and 1 ug/mL Ionomycin for 24 hours. Cells from Donors 4-5 were cultured in ImmunoCult™-XF T Cell Expansion Medium (Catalog #10981), with (activated) or without (control) ImmunoCult™ Human CD3/CD28 T Cell Activator (Catalog #10971) for 48 hours. Supernatants were collected from the cultures, and concentrations of secreted cytokines were determined using the Human IL-2 ELISA Kit (Catalog #02006). Activation by either PMA and Ionomycin or ImmunoCult™ Human CD3/CD28 T Cell Activator led to increased secretion of IL-2 compared to unstimulated control cultures. (B) Human B cells (Donors 6 - 11) cryopreserved in CryoStor®CS10 were thawed and activated with 1 µg/mL CD40 and 100 ng/mL IL-21 for 7 days. Supernatants were collected from the cultures and immunoglobulin G (IgG) production was measured using the Human IgG ELISA Antibody Pair Kit (Catalog #01994). Compared to unstimulated control cultures, B cell activation led to increased IgG secretion.
Publications (61)
Abstract
Cell stem cell 2018 MAY Super-Obese Patient-Derived iPSC Hypothalamic Neurons Exhibit Obesogenic Signatures and Hormone Responses.
Abstract
Stem cell reports
2018
MAY
Inflammatory Responses and Barrier Function of Endothelial Cells Derived from Human Induced Pluripotent Stem Cells.
Abstract
The Journal of clinical investigation
2018
JUN
Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment.
Abstract
Arthritis research & therapy
2018
JUL
Methods for high-dimensonal analysis of cells dissociated from cyropreserved synovial tissue.
Abstract
Oncotarget
2018
JAN
CD34- human placenta-derived mesenchymal stem cells protect against heat stroke mortality in rats.

 网站首页
网站首页