概要
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human Plasmacytoid DC Enrichment kit | 19062 | All | English |
| Product Information Sheet | RoboSep™ Human Plasmacytoid DC Enrichment Kit with Filter Tips | 19062RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human Plasmacytoid DC Enrichment kit | 19062 | All | English |
| Safety Data Sheet 2 | EasySep™ Human Plasmacytoid DC Enrichment kit | 19062 | All | English |
| Safety Data Sheet 3 | EasySep™ Human Plasmacytoid DC Enrichment kit | 19062 | All | English |
| Safety Data Sheet 4 | EasySep™ Human Plasmacytoid DC Enrichment kit | 19062 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human Plasmacytoid DC Enrichment Kit with Filter Tips | 19062RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human Plasmacytoid DC Enrichment Kit with Filter Tips | 19062RF | All | English |
| Safety Data Sheet 3 | RoboSep™ Human Plasmacytoid DC Enrichment Kit with Filter Tips | 19062RF | All | English |
| Safety Data Sheet 4 | RoboSep™ Human Plasmacytoid DC Enrichment Kit with Filter Tips | 19062RF | All | English |
数据及文献
Data
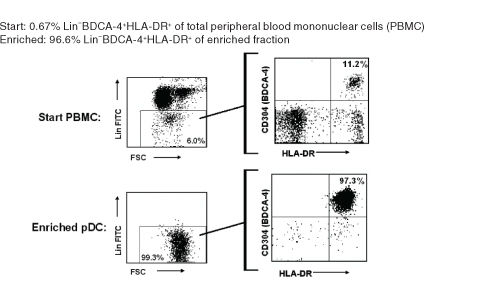
Figure 1. Typical EasySep™ Human pDC Enrichment Profile
Starting with 0.2 - 0.9% pDC in PBMC, the pDC content of the enriched fraction typically ranges from 87 - 97% purity based on the pDC phenotype of Lineage (CD3, CD14, CD16, CD19, CD20, CD34, CD56) negative, HLA-DR positive, and CD304 (BDCA-4) positive.
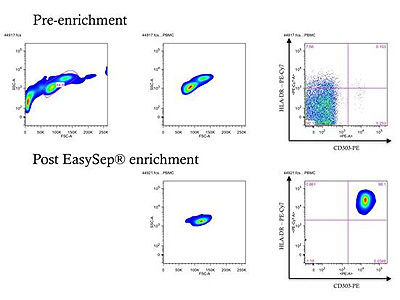
Figure 2. FACS Purity Data from pDC Kit User
FACS enrichment plots from Dr. Stuart R. McGregor Dallas of Princeton University. Prior to enrichment, the percentage of pDCs in total PBMC is approximately 0.1% (upper row), however following enrichment, a population of pDCs in excess of 97% pure can be obtained (lower row). pDCs identified using surface marker staining for CD303 and HLA-DR. Data originally posted as part of a product review in Biocompare.

 网站首页
网站首页