概要
For even faster cell isolations, we recommend the new EasySep™ Human NK Cell Isolation Kit (17955) which isolates cells in just 8 minutes.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | EasySep™ Human NK Cell Enrichment Kit | 19055 | All | English |
| Product Information Sheet | RoboSep™ Human NK Cell Enrichment Kit with Filter Tips | 19055RF | All | English |
| Safety Data Sheet | EasySep™ Human NK Cell Enrichment Kit | 19055 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human NK Cell Enrichment Kit with Filter Tips | 19055RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human NK Cell Enrichment Kit with Filter Tips | 19055RF | All | English |
数据及文献
Data
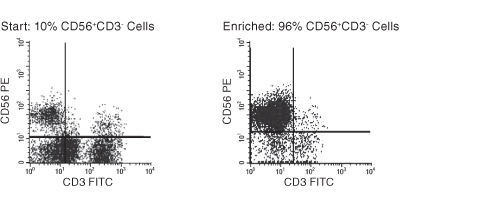
Figure 1. FACS Profile Results With EasySep™ Human NK Cell Enrichment Kit
The NK cell content of the enriched fraction varies, depending on the starting sample. Starting with previously frozen mononuclear cells containing more than 10% NK cells, the NK cell content of the enriched fraction typically ranges from 73% - 95%. Purities may be lower when starting with samples containing less than 10% NK cells.

 网站首页
网站首页