概要
The EasySep™ Human CD56 Positive Selection Kit II is designed to isolate CD56+ cells from fresh or previously frozen peripheral blood mononuclear cells and human skeletal muscle (myoblasts and fibroblasts) cultures by positive selection. Desired cells are targeted with antibody complexes recognizing CD56 and dextran-coated magnetic particles. The cocktail also contains an antibody to human Fc receptor to minimize nonspecific binding. Labeled cells are separated using an EasySep™ magnet without the use of columns. Cells of interest remain in the tube while unwanted cells are poured off. The CD56 antigen is expressed on NK cells, NKT cells and human myoblasts.
This product replaces the EasySep™ Human CD56 Positive Selection Kit (Catalog #18055) for even faster cell isolations.
This product replaces the EasySep™ Human CD56 Positive Selection Kit (Catalog #18055) for even faster cell isolations.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | EasySep™ Human CD56 Positive Selection Kit II | 17855 | All | English |
| Product Information Sheet 2 | EasySep™ Human CD56 Positive Selection Kit II | 17855 | All | English |
| Product Information Sheet | RoboSep™ Human CD56 Positive Selection Kit II | 17855RF | All | English |
| Safety Data Sheet 1 | EasySep™ Human CD56 Positive Selection Kit II | 17855 | All | English |
| Safety Data Sheet 2 | EasySep™ Human CD56 Positive Selection Kit II | 17855 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human CD56 Positive Selection Kit II | 17855RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human CD56 Positive Selection Kit II | 17855RF | All | English |
数据及文献
Data
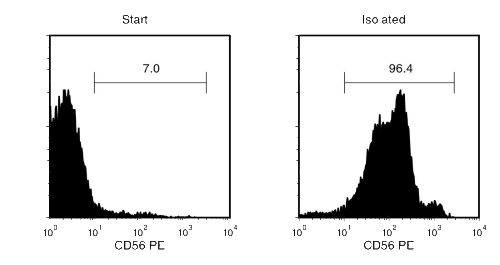
Figure 1. Typical EasySep™ Human CD56 Isolation Profile
Starting with human PBMCs, the CD56+ cell content of the isolated fraction is typically 94 ± 3% (mean ± SD, “The Big Easy” EasySep™ Magnet).
Publications (2)
Nature communications 2019 feb
PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals.
Abstract
Abstract
HIV persists in latently infected CD4+ T cells during antiretroviral therapy (ART). Immune checkpoint molecules, including PD-1, are preferentially expressed at the surface of persistently infected cells. However, whether PD-1 plays a functional role in HIV latency and reservoir persistence remains unknown. Using CD4+ T cells from HIV-infected individuals, we show that the engagement of PD-1 inhibits viral production at the transcriptional level and abrogates T-cell receptor (TCR)-induced HIV reactivation in latently infected cells. Conversely, PD-1 blockade with the monoclonal antibody pembrolizumab enhances HIV production in combination with the latency reversing agent bryostatin without increasing T cell activation. Our results suggest that the administration of immune checkpoint blockers to HIV-infected individuals on ART may facilitate latency disruption.
The Journal of clinical investigation 2019 dec
Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations.
Abstract
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disease with no known cause or mechanism. There is an increasing appreciation for the role of immune and metabolic dysfunction in the disease. ME/CFS has historically presented in outbreaks, often has a flu-like onset, and results in inflammatory symptoms. Patients suffer from severe fatigue and post-exertional malaise. There is little known about the metabolism of specific immune cells in ME/CFS patients. To investigate immune metabolism in ME/CFS, we isolated CD4+ and CD8+ T cells from 53 ME/CFS patients and 45 healthy controls. We analyzed glycolysis and mitochondrial respiration in resting and activated T cells, along with markers related to cellular metabolism, and plasma cytokines. We found that ME/CFS CD8+ T cells have reduced mitochondrial membrane potential compared to healthy controls. Both CD4+ and CD8+ T cells from ME/CFS patients had reduced glycolysis at rest, while CD8+ T cells also had reduced glycolysis following activation. ME/CFS patients had significant correlations between measures of T cell metabolism and plasma cytokine abundance that differed from healthy control subjects. Our data indicate that patients have impaired T cell metabolism consistent with ongoing immune alterations in ME/CFS that may illuminate the mechanism behind this disease.

 网站首页
网站首页