概要
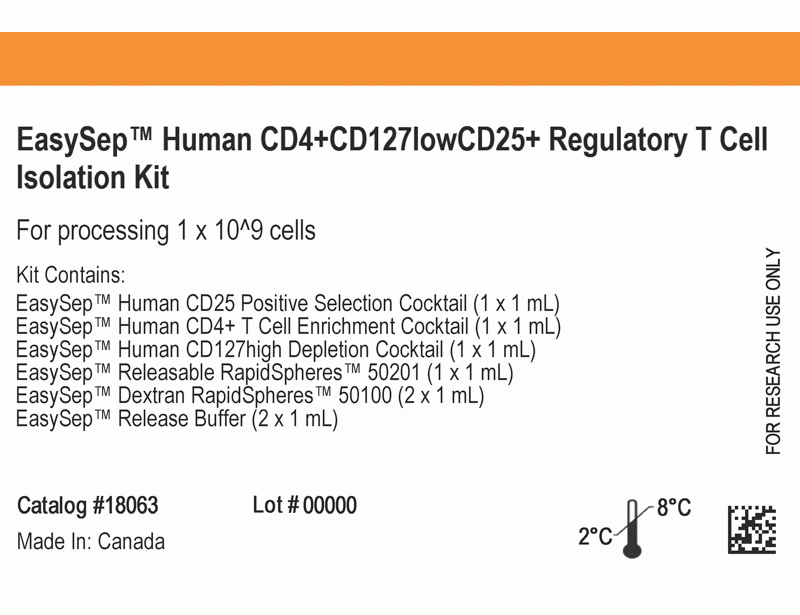
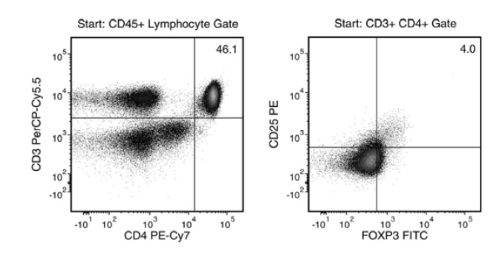
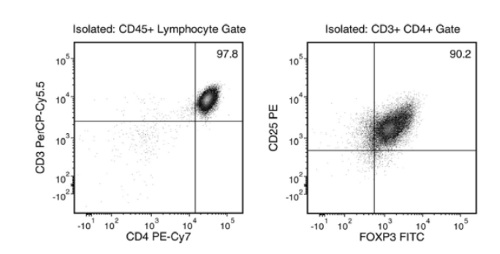
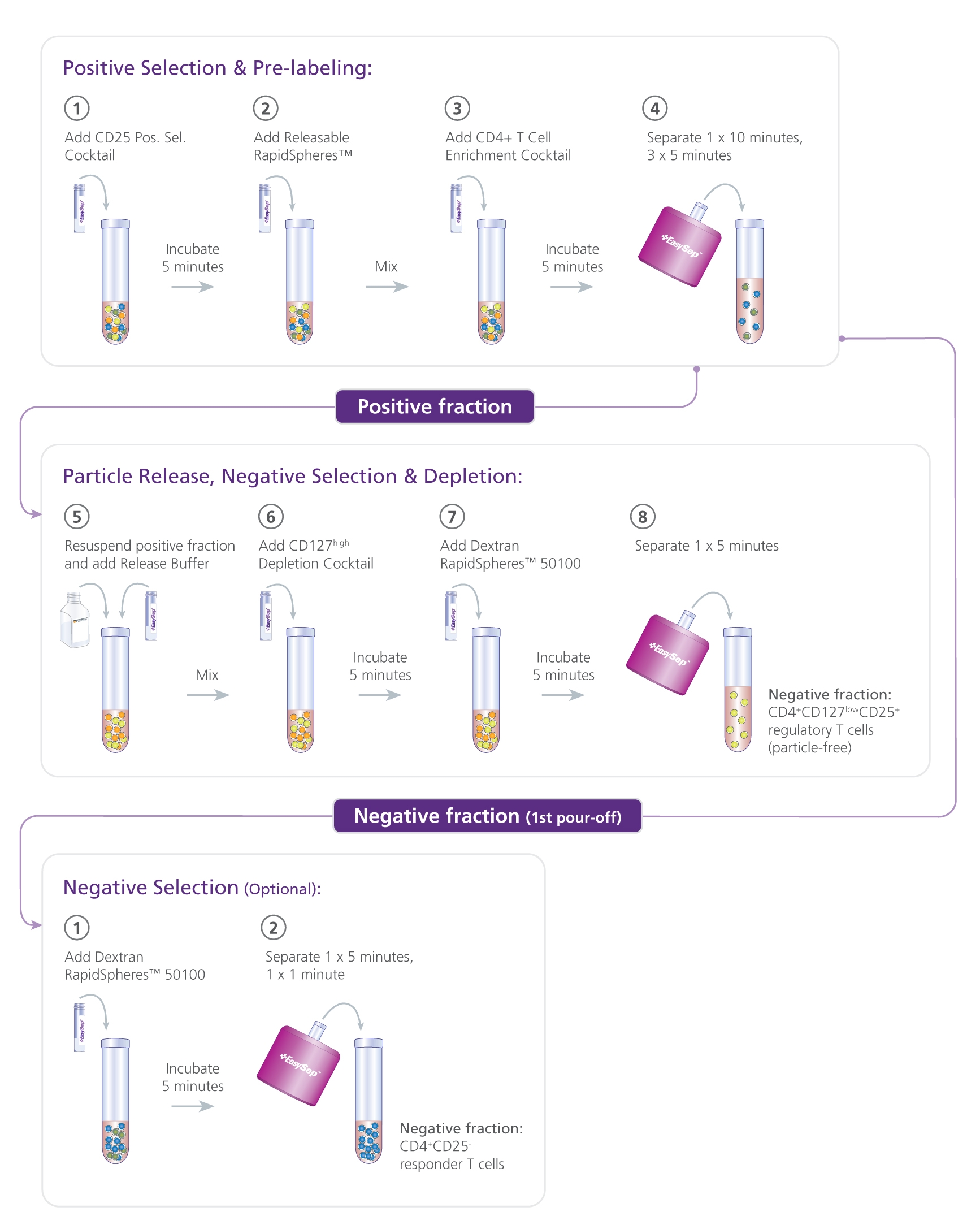
This kit isolates human CD4+CD127lowCD25+ regulatory T cells (Tregs) from fresh peripheral blood mononuclear cells (PBMCs) or leukapheresis samples. First, CD25+ cells are isolated by column-free immunomagnetic positive selection using EasySep™ Releasable RapidSpheres™. Then, bound magnetic particles are removed from the EasySep™-isolated CD25+ cells, and unwanted non-Tregs are targeted for depletion. The final isolated fraction contains highly purified CD4+CD127lowCD25+ cells that express high levels of FOXP3 and are immediately ready for downstream applications. An optional protocol allows for the isolation of CD4+CD25- responder T cells in parallel for use in functional studies. Following cell isolation with this EasySep™ kit, antibody complexes remain bound to the cell surface and may interact with Brilliant Violet™ antibody conjugates, polyethylene glycol-modified proteins, or other chemically related ligands.
数据及文献
Publications (3)
Metabolism: clinical and experimental 2020
Mannose is an insulin-regulated metabolite reflecting whole-body insulin sensitivity in man.
E. Ferrannini et al.
Abstract
Mannose is a glucose-associated serum metabolite mainly released by the liver. Recent studies have shown several unexpected pleiotropic effects of mannose including increased regulatory T cells (Tregs), prevention of auto-immune disease and ability to reduce growth of human cancer cells. We have previously shown in large cohorts that elevated serum mannose levels are associated with future development of type 2 diabetes (T2D) and cardiovascular disease. However, potential direct effects of mannose on insulin sensitivity in vivo or in vitro are unknown. We here show that administration of mannose (0.1 g/kg BW twice daily) for one week in man did not elicit negative effects on meal-modified glucose tolerance, markers of inflammation or insulin levels. Tregs number and insulin signaling in human liver cells were unchanged. These data suggest that mannose is a marker, and not a mediator, of insulin resistance. To verify this, we examined serum mannose levels during long-term euglycemic hyperinsulinemic clamps in non-diabetic and T2D individuals. Mannose was reduced by insulin infusion in proportion to whole-body insulin sensitivity. Thus, mannose is a biomarker of insulin resistance which may be useful for the early identification of diabetic individuals with insulin resistance and increased risk of its complications.
Journal of immunology (Baltimore, Md. : 1950) 2016 JUL
Netrin-1 Augments Chemokinesis in CD4+ T Cells In Vitro and Elicits a Proinflammatory Response In Vivo.
Boneschansker L et al.
Abstract
Netrin-1 is a neuronal guidance cue that regulates cellular activation, migration, and cytoskeleton rearrangement in multiple cell types. It is a chemotropic protein that is expressed within tissues and elicits both attractive and repulsive migratory responses. Netrin-1 has recently been found to modulate the immune response via the inhibition of neutrophil and macrophage migration. However, the ability of Netrin-1 to interact with lymphocytes and its in-depth effects on leukocyte migration are poorly understood. In this study, we profiled the mRNA and protein expression of known Netrin-1 receptors on human CD4(+) T cells. Neogenin, uncoordinated-5 (UNC5)A, and UNC5B were expressed at low levels in unstimulated cells, but they increased following mitogen-dependent activation. By immunofluorescence, we observed a cytoplasmic staining pattern of neogenin and UNC5A/B that also increased following activation. Using a novel microfluidic assay, we found that Netrin-1 stimulated bidirectional migration and enhanced the size of migratory subpopulations of mitogen-activated CD4(+) T cells, but it had no demonstrable effects on the migration of purified CD4(+)CD25(+)CD127(dim) T regulatory cells. Furthermore, using a short hairpin RNA knockdown approach, we observed that the promigratory effects of Netrin-1 on T effectors is dependent on its interactions with neogenin. In the humanized SCID mouse, local injection of Netrin-1 into skin enhanced inflammation and the number of neogenin-expressing CD3(+) T cell infiltrates. Neogenin was also observed on CD3(+) T cell infiltrates within human cardiac allograft biopsies with evidence of rejection. Collectively, our findings demonstrate that Netrin-1/neogenin interactions augment CD4(+) T cell chemokinesis and promote cellular infiltration in association with acute inflammation in vivo.
PLoS One 2015
The Macrophage Galactose-Type C-Type Lectin (MGL) Modulates Regulatory T Cell Functions
Zizzari IG et al.
Abstract
Regulatory T cells (Tregs) are physiologically designed to prevent autoimmune disease and maintain self-tolerance. In tumour microenvironments, their presence is related to a poor prognosis, and they influence the therapeutic outcome due to their capacity to suppress the immune response by cell-cell contact and to release immunosuppressive cytokines. In this study, we demonstrate that Treg immunosuppressive activity can be modulated by the cross-linking between the CD45RA expressed by Tregs and the C-type lectin MGL. This specific interaction strongly decreases the immunosuppressive activity of Tregs, restoring the proliferative capacity of co-cultured T lymphocytes. This effect can be attributed to changes in CD45RA and TCR signalling through the inhibition of Lck and inactivation of Zap-70, an increase in the Foxp3 methylation status and, ultimately, the reduced production of suppressive cytokines. These results indicate a role of MGL as an immunomodulator within the tumour microenvironment interfering with Treg functions, suggesting its possible use in the design of anticancer vaccines.
View All Publications

 网站首页
网站首页