概要
The EasySep™ Human B Cell Isolation Kit is designed to isolate B cells from fresh or previously frozen peripheral blood mononuclear cells or washed leukapheresis samples by immunomagnetic negative selection. The EasySep™ procedure involves labeling unwanted cells with antibody complexes and magnetic particles. The magnetically labeled cells are separated from the untouched, desired cells by using an EasySep™ magnet and simply pouring or pipetting the desired cells into a new tube. This product can be used in place of the EasySep™ Human B Cell Enrichment Kit (Catalog #19054) for even faster cell isolations.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet 1 | EasySep™ Human B Cell Isolation Kit | 17954 | Purchase date 2020-04-07 and later | English |
| Product Information Sheet 2 | EasySep™ Human B Cell Isolation Kit | 17954 | All other lots | English |
| Product Information Sheet 1 | RoboSep™ Human B Cell Isolation Kit | 17954RF | All other lots | English |
| Product Information Sheet 2 | RoboSep™ Human B Cell Isolation Kit | 17954RF | Purchase date 2020-04-07 and later | English |
| Safety Data Sheet 1 | EasySep™ Human B Cell Isolation Kit | 17954 | All | English |
| Safety Data Sheet 2 | EasySep™ Human B Cell Isolation Kit | 17954 | All | English |
| Safety Data Sheet 3 | EasySep™ Human B Cell Isolation Kit | 17954 | All | English |
| Safety Data Sheet 1 | RoboSep™ Human B Cell Isolation Kit | 17954RF | All | English |
| Safety Data Sheet 2 | RoboSep™ Human B Cell Isolation Kit | 17954RF | All | English |
| Safety Data Sheet 3 | RoboSep™ Human B Cell Isolation Kit | 17954RF | All | English |
数据及文献
Data
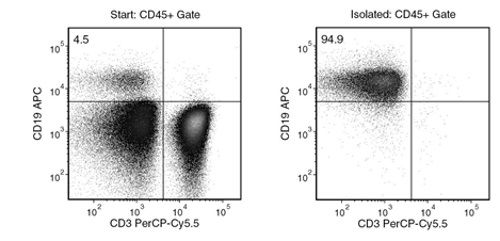
Figure 1. Typical EasySep™ Human B Cell Isolation Profile
Starting with human PBMCs, the B cell (CD3-CD19+) content of the isolated fraction is typically 95.1 ± 1.4% (mean ± SD). In the example above, the final purities of the start and isolated fractions are 4.5% and 94.9%, respectively.
Publications (6)
Journal of clinical medicine 2020 may
Inflammation-Induced Mucosal KYNU Expression Identifies Human Ileal Crohn's Disease.
Abstract
Abstract
The widely varying therapeutic response of patients with inflammatory bowel disease (IBD) continues to raise questions regarding the unclarified heterogeneity of pathological mechanisms promoting disease progression. While biomarkers for the differentiation of Crohn's disease (CD) versus ulcerative colitis (UC) have been suggested, specific markers for a CD subclassification in ileal CD versus colonic CD are still rare. Since an altered signature of the tryptophan metabolism is associated with chronic inflammatory disease, we sought to characterize potential biomarkers by focusing on the downstream enzymes and metabolites of kynurenine metabolism. Using immunohistochemical stainings, we analyzed and compared the mucosal tryptophan immune metabolism in bioptic samples from patients with active inflammation due to UC or CD versus healthy controls. Localization-specific quantification of immune cell infiltration, tryptophan-metabolizing enzyme expression and mucosal tryptophan downstream metabolite levels was performed. We found generally increased immune cell infiltrates in the tissue of all patients with IBD. However, in patients with CD, significant differences were found between regulatory T cell and neutrophil granulocyte infiltration in the ileum compared with the colon. Furthermore, we observed decreased kynurenine levels as well as strong kynureninase (KYNU) expression specifically in patients with ileal CD. Correspondingly, significantly elevated levels of the kynurenine metabolite 3-hydroxyanthranilic acid were detected in the ileal CD samples. Highlighting the heterogeneity of the different phenotypes of CD, we identified KYNU as a potential mucosal biomarker allowing the localization-specific differentiation of ileal CD versus colonic CD.
Journal of autoimmunity 2019 may
Transitional B cells in quiescent SLE: An early checkpoint imprinted by IFN.
Abstract
Abstract
Systemic lupus (SLE) is characterized by a break of B cell tolerance that plays a central role in disease pathophysiology. An early checkpoint defect occurs at the transitional stage leading to the survival of autoreactive B cells and consequently the production of pathogenic autoantibodies. The main purpose of our work was to determine whether transitional B cells, as the most immature na{\{i}}ve B cell subset upstream of pathogenic B cells display specific features compared to healthy non SLE subjects. Through extensive analysis of transitional B cells from untreated or low treated mostly Caucasian SLE patients we demonstrated that transitional (T1 and T2) B cell frequencies were increased in SLE and positively correlated with disease activity. SLE transitional B cells displayed defects in two closely inter-related molecules (i.e. TLR9 defective responses and CD19 downregulation). RNA sequencing of sorted transitional B cells from untreated patients revealed a predominant overexpression of interferon stimulated genes (ISGs) even out of flares. In addition early transitional B cells from the bone marrow displayed the highest interferon score reflecting a B cell interferon burden of central origin. Hence the IFN signature in transitional B cells is not confined to African American SLE patients and exists in quiescent disease since the medullary stage. These results suggest that in SLE these 3 factors (i.e. IFN imprintment CD19 downregulation and TLR9 responses impairment) could take part at the early transitional B cell stage in B cell tolerance by-pass ultimately leading in periphery to the expansion of autoantibodies-secreting cells."
Journal of autoimmunity 2019 jul
Abatacept modulates CD80 and CD86 expression and memory formation in human B-cells.
Abstract
Abstract
BACKGROUND Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits T-cell activation and is expressed on T-regulatory cells. Human CTLA-4 deficiency results in severe immune dysregulation. Abatacept (CTLA-4 Ig) is approved for the treatment of rheumatoid arthritis (RA) and its mechanism of action is attributed to effects on T-cells. It is known that CTLA-4 modulates the expression of its ligands CD80 and CD86 on antigen presenting cells (APC) by transendocytosis. As B-cells express CD80/CD86 and function as APC, we hypothesize that B-cells are a direct target of abatacept. OBJECTIVES To investigate direct effects of abatacept on human B-lymphocytes in vitro and in RA patients. METHODS The effect of abatacept on healthy donor B-cells' phenotype, activation and CD80/CD86 expression was studied in vitro. Nine abatacept-treated RA patients were studied. Seven of these were followed up to 24 months, and two up to 12 months only and treatment response, immunoglobulins, ACPA, RF concentrations, B-cell phenotype and ACPA-specific switched memory B-cell frequency were assessed. RESULTS B-cell development was unaffected by abatacept. Abatacept treatment resulted in a dose-dependent decrease of CD80/CD86 expression on B-cells in vitro, which was due to dynamin-dependent internalization. RA patients treated with abatacept showed a progressive decrease in plasmablasts and serum IgG. While ACPA-titers only moderately declined, the frequency of ACPA-specific switched memory B-cells significantly decreased. CONCLUSIONS Abatacept directly targets B-cells by reducing CD80/CD86 expression. Impairment of antigen presentation and T-cell activation may result in altered B-cell selection, providing a new therapeutic mechanism and a base for abatacept use in B-cell mediated autoimmunity.
Scientific reports 2019 jan
BCR-associated factors driving chronic lymphocytic leukemia cells proliferation ex vivo.
Abstract
Abstract
A chronic antigenic stimulation is believed to sustain the leukemogenic development of chronic lymphocytic leukemia (CLL) and most of lymphoproliferative malignancies developed from mature B cells. Reproducing a proliferative stimulation ex vivo is critical to decipher the mechanisms of leukemogenesis in these malignancies. However, functional studies of CLL cells remains limited since current ex vivo B cell receptor (BCR) stimulation protocols are not sufficient to induce the proliferation of these cells, pointing out the need of mandatory BCR co-factors in this process. Here, we investigated benefits of several BCR co-stimulatory molecules (IL-2, IL-4, IL-15, IL-21 and CD40 ligand) in multiple culture conditions. Our results demonstrated that BCR engagement (anti-IgM ligation) concomitant to CD40 ligand, IL-4 and IL-21 stimulation allowed CLL cells proliferation ex vivo. In addition, we established a proliferative advantage for ZAP70 positive CLL cells, associated to an increased phosphorylation of ZAP70/SYK and STAT6. Moreover, the use of a tri-dimensional matrix of methylcellulose and the addition of TLR9 agonists further increased this proliferative response. This ex vivo model of BCR stimulation with T-derived cytokines is a relevant and efficient model for functional studies of CLL as well as lymphoproliferative malignancies.
Frontiers in immunology 2018
CD24 Expression and B Cell Maturation Shows a Novel Link With Energy Metabolism: Potential Implications for Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
Abstract
Abstract
CD24 expression on pro-B cells plays a role in B cell selection and development in the bone marrow. We previously detected higher CD24 expression and frequency within IgD+ na{\{i}}ve and memory B cells in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) compared with age-matched healthy controls (HC). Here we investigated the relationship between CD24 expression and B cell maturation. In vitro stimulation of isolated B cells in response to conventional agonists were used to follow the dynamics of CD24 positivity during proliferation and differentiation (or maturation). The relationship between CD24 expression to cycles of proliferation and metabolism in purified B cells from HC was also investigated using phospho-flow (phosphorylation of AMPK-pAMPK) 1proton nuclear magnetic resonance and Mitotracker Far-red (Mitochondrial mass-MM). In vitro in the absence of stimulation there was an increased percentage of CD24+ viable B cells in ME/CFS patients compared to HC (p {\textless} 0.05) following 5 days culture. Following stimulation with B cell agonists percentage of CD24+B cells in both na{\"{i}}ve and memory B cell populations decreased. P {\textless} 0.01). There was a negative relationship between percentage of CD24+B cells with MM (R2 = 0.76; p {\textless} 0.01) which was subsequently lost over sequential cycles of proliferation. There was a significant correlation between CD24 expression on B cells and the usage of glucose and secretion of lactate in vitro. Short term ligation of the B cell receptor with anti-IgM antibody significantly reduced the viability of CD24+ memory B cells compared to those cross-linked by anti-IgD or anti-IgG antibody. A clear difference was found between na{\""{i}}ve and memory B cells with respect to CD24 expression and pAMPK most notably a strong positive association in IgD+IgM+ memory B cells. In vitro findings confirmed dysregulation of CD24-expressing B cells from ME/CFS patients previously suggested by immunophenotype studies of B cells from peripheral blood. CD24-negative B cells underwent productive proliferation whereas CD24+ B cells were either unresponsive or susceptible to cell death upon BCR-engagement alone. We suggest that CD24 expression may reflect variations in energy metabolism on different B cell subsets."""
Journal of Immunology 2016 MAR
LLT1 and CD161 Expression in Human Germinal Centers Promotes B Cell Activation and CXCR4 Downregulation.
Abstract
Abstract
Germinal centers (GCs) are microanatomical structures critical for the development of high-affinity Abs and B cell memory. They are organized into two zones, light and dark, with coordinated roles, controlled by local signaling. The innate lectin-like transcript 1 (LLT1) is known to be expressed on B cells, but its functional role in the GC reaction has not been explored. In this study, we report high expression of LLT1 on GC-associated B cells, early plasmablasts, and GC-derived lymphomas. LLT1 expression was readily induced via BCR, CD40, and CpG stimulation on B cells. Unexpectedly, we found high expression of the LLT1 ligand, CD161, on follicular dendritic cells. Triggering of LLT1 supported B cell activation, CD83 upregulation, and CXCR4 downregulation. Overall, these data suggest that LLT1-CD161 interactions play a novel and important role in B cell maturation within the GC in humans.

 网站首页
网站首页