概要
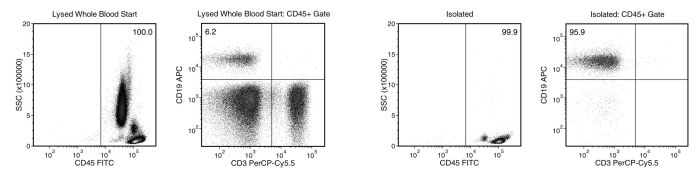
Isolate highly purified B cells directly from human whole blood without the need for density gradient centrifugation, RBC sedimentation or lysis. This platform provides fast and fully immunomagnetic negative selection.
This cell isolation kit targets non-B cells for removal with EasySep™ Direct RapidSpheres™ and antibodies recognizing specific surface antigens. Unwanted cells are separated from desired cells using an EasySep™ magnet, and untouched target cells are then pipetted or poured into a new tube.
Isolated cells can be used directly in downstream applications such as flow cytometry, culture or DNA/RNA extraction.
数据及文献
Publications (2)
mAbs 2016 JUL
A novel antibody discovery platform identifies anti-influenza A broadly neutralizing antibodies from human memory B cells.
Xiao X et al.
Abstract
Monoclonal antibody isolation directly from circulating human B cells is a powerful tool to delineate humoral responses to pathological conditions and discover antibody therapeutics. We have developed a platform aimed at improving the efficiencies of B cell selection and V gene recovery. Here, memory B cells are activated and amplified using Epstein-Barr virus infection, co-cultured with CHO-muCD40L cells, and then assessed by functional screenings. An in vitro transcription and translation (IVTT) approach was used to analyze variable (V) genes recovered from each B cell sample and identify the relevant heavy/light chain pair(s). We achieved efficient amplification and activation of memory B cells, and eliminated the need to: 1) seed B cells at clonal level (≤1 cell/well) or perform limited dilution cloning; 2) immortalize B cells; or 3) assemble V genes into an IgG expression vector to confirm the relevant heavy/light chain pairing. Cross-reactive antibodies targeting a conserved epitope on influenza A hemagglutinin were successfully isolated from a healthy donor. In-depth analysis of the isolated antibodies suggested their potential uses as anti-influenza A antibody therapeutics and uncovered a distinct affinity maturation pathway. Importantly, our results showed that cognate heavy/light chain pairings contributed to both the expression level and binding abilities of our newly isolated VH1-69 family, influenza A neutralizing antibodies, contrasting with previous observations that light chains do not significantly contribute to the function of this group of antibodies. Our results further suggest the potential use of the IVTT as a powerful antibody developability assessment tool.
Journal of immunology (Baltimore, Md. : 1950) 2016 JUL
FcγRIIB-Independent Mechanisms Controlling Membrane Localization of the Inhibitory Phosphatase SHIP in Human B Cells.
Pauls SD et al.
Abstract
SHIP is an important regulator of immune cell signaling that functions to dephosphorylate the phosphoinositide phosphatidylinositol 3,4,5-trisphosphate at the plasma membrane and mediate protein-protein interactions. One established paradigm for SHIP activation involves its recruitment to the phospho-ITIM motif of the inhibitory receptor FcγRIIB. Although SHIP is essential for the inhibitory function of FcγRIIB, it also has critical modulating functions in signaling initiated from activating immunoreceptors such as B cell Ag receptor. In this study, we found that SHIP is indistinguishably recruited to the plasma membrane after BCR stimulation with or without FcγRIIB coligation in human cell lines and primary cells. Interestingly, fluorescence recovery after photobleaching analysis reveals differential mobility of SHIP-enhanced GFP depending on the mode of stimulation, suggesting that although BCR and FcγRIIB can both recruit SHIP, this occurs via distinct molecular complexes. Mutagenesis of a SHIP-enhanced GFP fusion protein reveals that the SHIP-Src homology 2 domain is essential in both cases whereas the C terminus is required for recruitment via BCR stimulation, but is less important with FcγRIIB coligation. Experiments with pharmacological inhibitors reveal that Syk activity is required for optimal stimulation-induced membrane localization of SHIP, whereas neither PI3K or Src kinase activity is essential. BCR-induced association of SHIP with binding partner Shc1 is dependent on Syk, as is tyrosine phosphorylation of both partners. Our results indicate that FcγRIIB is not uniquely able to promote membrane recruitment of SHIP, but rather modulates its function via formation of distinct signaling complexes. Membrane recruitment of SHIP via Syk-dependent mechanisms may be an important factor modulating immunoreceptor signaling.
View All Publications

 网站首页
网站首页



