概要
The RosetteSep™ Human Bone Marrow Progenitor Cell Pre-Enrichment Cocktail is designed to pre-enrich progenitor cells by removing lineage-specific cells from whole bone marrow by negative selection. Unwanted cells are targeted for removal with Tetrameric Antibody Complexes recognizing CD3, CD11b, CD14, CD16, CD19, CD56, CD66b and glycophorin A on red blood cells (RBCs). When centrifuged over a buoyant density medium such as Lymphoprep™ (Catalog #07801), the unwanted cells pellet along with the RBCs. The pre-enriched progenitor cells are present as an enriched population at the interface between the plasma and the buoyant density medium.
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | RosetteSep™ Human Bone Marrow Progenitor Cell Pre-Enrichment Cocktail | 15027, 15067 | All | English |
| Safety Data Sheet | RosetteSep™ Human Bone Marrow Progenitor Cell Pre-Enrichment Cocktail | 15027 | All | English |
数据及文献
Data
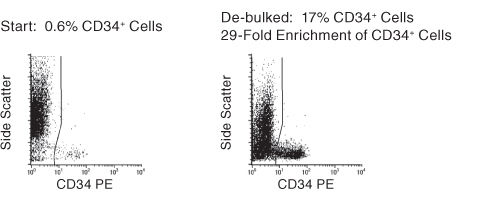
Figure 1. FACS Profile Results With RosetteSep™ Human Bone Marrow Progenitor Cell Pre-Enrichment Kit
Results (mean ± 1 S.D.): Enrichment of CD34+ Cells: 25 ± 10%. Fold Enrichment of CFC: 10-53 Fold
Publications (2)
Stem cells (Dayton, Ohio) 2006 NOV
Growth of mesenchymal stem cells on electrospun type I collagen nanofibers.
Abstract
Abstract
We reconstituted type I collagen nanofibers prepared by electrospin technology and examined the morphology, growth, adhesion, cell motility, and osteogenic differentiation of human bone marrow-derived mesenchymal stem cells (MSCs) on three nano-sized diameters (50-200, 200-500, and 500-1,000 nm). Results from scanning electron microscopy showed that cells on the nanofibers had a more polygonal and flattened cell morphology. MTS (3-[4,5-dimethythiazol-2-yl]-5-[3-carboxy-methoxyphenyl]-2-[4-sul-fophenyl]-2H-tetrazolium compound) assay demonstrated that the MSCs grown on 500-1,000-nm nanofibers had significantly higher cell viability than the tissue culture polystyrene control. A decreased amount of focal adhesion formation was apparent in which quantifiable staining area of the cytoplasmic protein vinculin for the 200-500-nm nanofibers was 39% less compared with control, whereas the area of quantifiable vinculin staining was 45% less for both the 200-500-nm and 500-1,000-nm nanofibers. The distances of cell migration were quantified on green fluorescent protein-nucleofected cells and was 56.7%, 37.3%, and 46.3% for 50-200, 200-500, and 500-1,000 nm, respectively, compared with those on the control. Alkaline phosphatase activity demonstrated no differences after 12 days of osteogenic differentiation, and reverse transcription-polymerase chain reaction (RT-PCR) analysis showed comparable osteogenic gene expression of osteocalcin, osteonectin, and ostepontin between cells differentiated on polystyrene and nanofiber surfaces. Moreover, single-cell RT-PCR of type I collagen gene expression demonstrated higher expression on cells seeded on the nanofibers. Therefore, type I collagen nanofibers support the growth of MSCs without compromising their osteogenic differentiation capability and can be used as a scaffold for bone tissue engineering to facilitate intramembranous bone formation. Further efforts are necessary to enhance their biomimetic properties.
Blood 2004 NOV
Susceptibility of human fetal mesenchymal stem cells to Kaposi sarcoma-associated herpesvirus.
Abstract
Abstract
Recent reports link Kaposi sarcoma-associated herpesvirus (KSHV) infection of bone marrow cells to bone marrow failure and lymphoproliferative syndromes. The identity of the infected marrow cells, however, remains unclear. Other work has demonstrated that circulating mononuclear cells can harbor KSHV where its detection predicts the onset and severity of Kaposi sarcoma. In either setting, bone marrow precursors may serve as viral reservoirs. Since mesenchymal stem cells (MSCs) in human bone marrow regulate the differentiation and proliferation of adjacent hematopoietic precursors, we investigated their potential role in KSHV infection. Our results indicate that primary MSCs are susceptible to both cell-free and cell-associated KSHV in culture. Moreover, infection persisted within nearly half of the cells for up to 6 weeks. Thus, MSCs possess a clear capacity to support KSHV infection and warrant further exploration into their potential role in KSHV-related human disease.

 网站首页
网站首页





