概要
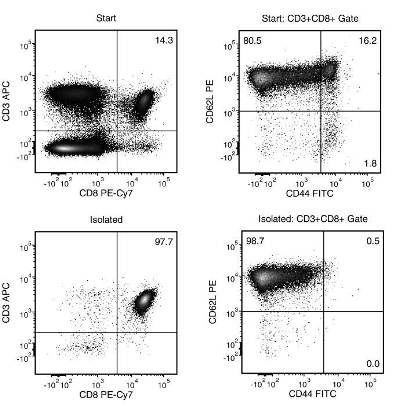
The EasySep™ Mouse Naïve CD8+ T Cell Isolation Kit is designed to isolate naïve CD62L+CD44-CD8+ T cells from single-cell suspensions of splenocytes or lymph nodes by negative selection. Unwanted cells are targeted for removal with biotinylated antibodies that are directed against non-naïve CD8+ T cells (CD4, CD11b, CD11c, CD19, CD44, CD45R/B220, CD49b, TCRγ/δ, TER119) and streptavidin-coated magnetic particles. Labeled cells are separated using an EasySep™ magnet without the use of columns. Desired cells are poured off into a new tube.
数据及文献
Publications (4)
Nature microbiology 2019 may
Direct interaction of whole-inactivated influenza A and pneumococcal vaccines enhances influenza-specific immunity.
S. C. David et al.
Abstract
The upper respiratory tract is continuously exposed to a vast array of potentially pathogenic viruses and bacteria. Influenza A virus (IAV) has particular synergism with the commensal bacterium Streptococcus pneumoniae in this niche, and co-infection exacerbates pathogenicity and causes significant mortality. However, it is not known whether this synergism is associated with a direct interaction between the two pathogens. We have previously reported that co-administration of a whole-inactivated IAV vaccine (gamma-Flu) with a whole-inactivated pneumococcal vaccine (gamma-PN) enhances pneumococcal-specific responses. In this study, we show that mucosal co-administration of gamma-Flu and gamma-PN similarly augments IAV-specific immunity, particularly tissue-resident memory cell responses in the lung. In addition, our in vitro analysis revealed that S. pneumoniae directly interacts with both gamma-Flu and with live IAV, facilitating increased uptake by macrophages as well as increased infection of epithelial cells by IAV. These observations provide an additional explanation for the synergistic pathogenicity of IAV and S. pneumoniae, as well as heralding the prospect of exploiting the phenomenon to develop better vaccine strategies for both pathogens.
Journal of immunology (Baltimore, Md. : 1950) 2016 NOV
Autocrine Type I IFN Signaling in Dendritic Cells Stimulated with Fungal β-Glucans or Lipopolysaccharide Promotes CD8 T Cell Activation.
Hassanzadeh-Kiabi N et al.
Abstract
Type I IFNs are key mediators of immune defense against viruses and bacteria. Type I IFNs were also previously implicated in protection against fungal infection, but their roles in antifungal immunity have not been thoroughly investigated. A recent study demonstrated that bacterial and fungal β-glucans stimulate IFN-β production by dendritic cells (DCs) following detection by the Dectin-1 receptor, but the effects of β-glucan-induced type I IFNs have not been defined. We investigated whether type I IFNs regulate CD8 T cell activation by fungal β-glucan particle-stimulated DCs. We demonstrate that β-glucan-stimulated DCs induce CD8 T cell proliferation, activation marker (CD44 and CD69) expression, and production of IFN-γ, IL-2, and granzyme B. Moreover, we show that type I IFNs support robust CD8 T cell activation (proliferation and IFN-γ and granzyme B production) by β-glucan-stimulated DCs in vitro and in vivo due to autocrine effects on the DCs. Specifically, type I IFNs promote Ag presentation on MHC I molecules, CD86 and CD40 expression, and the production of IL-12 p70, IL-2, IL-6, and TNF-α by β-glucan-stimulated DCs. We also demonstrate a role for autocrine type I IFN signaling in bacterial LPS-induced DC maturation, although, in the context of LPS stimulation, this mechanism is not so critical for CD8 T cell activation (promotes IFN-γ production but not proliferation or granzyme B production). This study provides insight into the mechanisms underlying CD8 T cell activation during infection, which may be useful in the rational design of vaccines directed against pathogens and tumors.
Cell Reports 2016 FEB
Mammalian Target of Rapamycin Complex 2 Controls CD8 T Cell Memory Differentiation in a Foxo1-Dependent Manner.
Zhang L et al.
Abstract
Upon infection, antigen-specific naive CD8 T cells are activated and differentiate into short-lived effector cells (SLECs) and memory precursor cells (MPECs). The underlying signaling pathways remain largely unresolved. We show that Rictor, the core component of mammalian target of rapamycin complex 2 (mTORC2), regulates SLEC and MPEC commitment. Rictor deficiency favors memory formation and increases IL-2 secretion capacity without dampening effector functions. Moreover, mTORC2-deficient memory T cells mount more potent recall responses. Enhanced memory formation in the absence of mTORC2 was associated with Eomes and Tcf-1 upregulation, repression of T-bet, enhanced mitochondrial spare respiratory capacity, and fatty acid oxidation. This transcriptional and metabolic reprogramming is mainly driven by nuclear stabilization of Foxo1. Silencing of Foxo1 reversed the increased MPEC differentiation and IL-2 production and led to an impaired recall response of Rictor KO memory T cells. Therefore, mTORC2 is a critical regulator of CD8 T cell differentiation and may be an important target for immunotherapy interventions.
Journal of Immunology 2016 FEB
Phosphatidylinositol 3-Kinase p110δ Isoform Regulates CD8+ T Cell Responses during Acute Viral and Intracellular Bacterial Infections.
Gracias DT et al.
Abstract
The p110δ isoform of PI3K is known to play an important role in immunity, yet its contribution to CTL responses has not been fully elucidated. Using murine p110δ-deficient CD8(+) T cells, we demonstrated a critical role for the p110δ subunit in the generation of optimal primary and memory CD8(+) T cell responses. This was demonstrated in both acute viral and intracellular bacterial infections in mice. We show that p110δ signaling is required for CD8(+) T cell activation, proliferation and effector cytokine production. We provide evidence that the effects of p110δ signaling are mediated via Akt activation and through the regulation of TCR-activated oxidative phosphorylation and aerobic glycolysis. In light of recent clinical trials that employ drugs targeting p110δ in certain cancers and other diseases, our study suggests caution in using these drugs in patients, as they could potentially increase susceptibility to infectious diseases. These studies therefore reveal a novel and direct role for p110δ signaling in in vivo CD8(+) T cell immunity to microbial pathogens.
View All Publications

 网站首页
网站首页